Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6
- PMID: 28815688
- PMCID: PMC5986189
- DOI: 10.1002/ajh.24888
Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6
Abstract
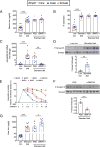
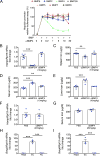
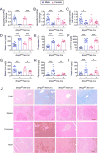
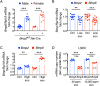
Hepcidin is a key iron regulatory hormone that controls expression of the iron exporter ferroportin to increase the iron supply when needed to support erythropoiesis and other essential functions, but to prevent the toxicity of iron excess. The bone morphogenetic protein (BMP)-SMAD signaling pathway, through the ligand BMP6 and the co-receptor hemojuvelin, is a central regulator of hepcidin transcription in the liver in response to iron. Here, we show that dietary iron loading has a residual ability to induce Smad signaling and hepcidin expression in Bmp6-/- mice, effects that are blocked by a neutralizing BMP2/4 antibody. Moreover, BMP2/4 antibody inhibits hepcidin expression and induces iron loading in wildtype mice, whereas a BMP4 antibody has no effect. Bmp2 mRNA is predominantly expressed in endothelial cells of the liver, where its baseline expression is higher, but its induction by iron is less robust than Bmp6. Mice with a conditional ablation of Bmp2 in endothelial cells exhibit hepcidin deficiency, serum iron overload, and tissue iron loading in liver, pancreas and heart, with reduced spleen iron. Together, these data demonstrate that in addition to BMP6, endothelial cell BMP2 has a non-redundant role in hepcidin regulation by iron.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
JLB has ownership interest in Ferrumax Pharmaceuticals, which has licensed technology from the Massachusetts General Hospital based on work cited here and in prior publications. All other authors have nothing to declare.
Figures
References
-
- de Benoist B, McLean E, Egli I, Cogswell M. WHO Global Database on Anaemia. Geneva: World Health Organization; 2008. World prevalence of anaemia 1993–2005. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_de...
-
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. - PubMed
-
- Pietrangelo A. Iron and the liver. Liver Int. 2016;36:116–123. - PubMed
-
- Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016;172:512–523. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
