First-line treatment selection and early monitoring patterns in chronic phase-chronic myeloid leukemia in routine clinical practice: SIMPLICITY
- PMID: 28815757
- PMCID: PMC5659133
- DOI: 10.1002/ajh.24887
First-line treatment selection and early monitoring patterns in chronic phase-chronic myeloid leukemia in routine clinical practice: SIMPLICITY
Abstract
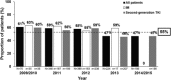
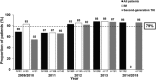
Achieving successful outcomes in chronic phase-chronic myeloid leukemia (CP-CML) requires careful monitoring of cytogenetic/molecular responses (CyR/MR). SIMPLICITY (NCT01244750) is an observational study exploring tyrosine kinase inhibitor use and management patterns in patients with CP-CML receiving first-line imatinib (n = 416), dasatinib (n = 418) or nilotinib (n = 408) in the US and 6 European countries in routine clinical practice. Twelve-month follow-up data of 1242 prospective patients (enrolled October 01 2010-September 02 2015) are reported. 81% of patients had baseline comorbidities. Treatment selection was based on perceived efficacy over patient comorbidity profile. There was a predominance of imatinib-treated patients enrolled earlier in the study, with subsequent shift toward dasatinib- and nilotinib-treated patients by 2013/2014. Monitoring for either CyR/MR improved over time and was documented for 36%, 82%, and 95% of patients by 3, 6, and 12 months, respectively; 5% had no documentation of CyR/MR monitoring during the first year of therapy. Documentation of MR/CyR testing was higher in Europe than the US (P < .001) and at academic versus community practices (P = .001). Age <65 years, patients being followed at sites within Europe, those followed at academic centers and patients no longer on first-line therapy were more likely to be monitored by 12 months. SIMPLICITY demonstrates that the NCCN and ELN recommendations on response monitoring have not been consistently translated into routine clinical practice. In the absence of appropriate monitoring practices, clinical response to TKI therapy cannot be established, any needed changes to treatment strategy will thus not be implemented, and long-term patient outcomes are likely to be impacted.
© 2017 The Authors American Journal of Hematology Published by Wiley Periodicals, Inc.
Figures
References
-
- Hochhaus A. Educational session: managing chronic myeloid leukemia as a chronic disease. Hematol Am Soc Hematol Educ Program. 2011;2011:128–135. - PubMed
-
- Gambacorti‐Passerini C, Antolini L, Mahon F‐X, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103:553–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
