Electrical stimulation of gut motility guided by an in silico model
- PMID: 28816177
- PMCID: PMC5724779
- DOI: 10.1088/1741-2552/aa86c8
Electrical stimulation of gut motility guided by an in silico model
Abstract
Objective: Neuromodulation of the central and peripheral nervous systems is becoming increasingly important for treating a diverse set of diseases-ranging from Parkinson's Disease and epilepsy to chronic pain. However, neuromodulation of the gastrointestinal (GI) tract has achieved relatively limited success in treating functional GI disorders, which affect a significant population, because the effects of stimulation on the enteric nervous system (ENS) and gut motility are not well understood. Here we develop an integrated neuromechanical model of the ENS and assess neurostimulation strategies for enhancing gut motility, validated by in vivo experiments.
Approach: The computational model included a network of enteric neurons, smooth muscle fibers, and interstitial cells of Cajal, which regulated propulsion of a virtual pellet in a model of gut motility.
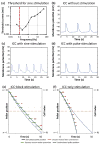
Main results: Simulated extracellular stimulation of ENS-mediated motility revealed that sinusoidal current at 0.5 Hz was more effective at increasing intrinsic peristalsis and reducing colon transit time than conventional higher frequency rectangular current pulses, as commonly used for neuromodulation therapy. Further analysis of the model revealed that the 0.5 Hz sinusoidal currents were more effective at modulating the pacemaker frequency of interstitial cells of Cajal. To test the predictions of the model, we conducted in vivo electrical stimulation of the distal colon while measuring bead propulsion in awake rats. Experimental results confirmed that 0.5 Hz sinusoidal currents were more effective than higher frequency pulses at enhancing gut motility.
Significance: This work demonstrates an in silico GI neuromuscular model to enable GI neuromodulation parameter optimization and suggests that low frequency sinusoidal currents may improve the efficacy of GI pacing.
Figures





References
-
- BORNSTEIN JC, FURNESS JB, KELLY HF, BYWATER RA, NEILD TO, BERTRAND PP. Computer simulation of the enteric neural circuits mediating an ascending reflex: roles of fast and slow excitatory outputs of sensory neurons. J Auton Nerv Syst. 1997;64:143–57. - PubMed
-
- BOSSETTI CA, BIRDNO MJ, GRILL WM. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J Neural Eng. 2008;5:44–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
