Efficacy and safety evaluation of fludarabine-based chemotherapy regimen for patients with non-Hodgkin lymphoma: A meta-analysis
- PMID: 28816963
- PMCID: PMC5571700
- DOI: 10.1097/MD.0000000000007781
Efficacy and safety evaluation of fludarabine-based chemotherapy regimen for patients with non-Hodgkin lymphoma: A meta-analysis
Abstract
This meta-analysis was performed to evaluate the efficacy and safety of fludarabine (F)-based regimen for the treatment of non-Hodgkin lymphoma (NHL) compared with other regimens with no F contained.PubMed, Embase, Cochrane Library, Wanfang, VIP, and CNKI databases were searched to identify eligible literatures. R software version 3.12 was used for statistical analysis. Odds ratio (OR) with 95% confidence interval (CI) were utilized to express the complete response, overall response and adverse events outcomes. Egger test was carried out to examine the publication bias and sensitivity analysis was performed to evaluate the stability of our results.Twelve eligible literatures consisting of 1587 patients were included in this study. Greater complete response (OR = 1.66, 95% CI: 0.98-2.80) and overall response (OR = 1.38, 95% CI: 0.85-2.24) were found for patients who received F-based regimen than those received other regimens, although the results were not statistically significant. In addition, F-based regimen was associated with significantly lower risk of adverse events compared with other regimens (OR = 0.46, 95% CI: 0.28-0.74). Results of subgroup analysis showed that significantly lower incidence was presented only for constipation among the 7 specific adverse events (OR = 0.03, 95% CI: 0.01-0.14).F-based chemotherapy regimen was an effective and well-tolerated treatment for patients with NHL.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
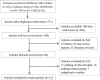


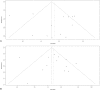

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

