A Phase 1 and 2 study of Filanesib alone and in combination with low-dose dexamethasone in relapsed/refractory multiple myeloma
- PMID: 28817190
- PMCID: PMC5856158
- DOI: 10.1002/cncr.30892
A Phase 1 and 2 study of Filanesib alone and in combination with low-dose dexamethasone in relapsed/refractory multiple myeloma
Abstract
Background: Filanesib (ARRY-520) is a highly selective inhibitor of kinesin spindle protein, which has demonstrated preclinical antimyeloma activity.
Methods: This open-label Phase 1/2 study determined the maximum tolerated dose of Filanesib administered on Days 1 and 2 of 14-Day Cycles in patients with multiple myeloma (MM) and included expansion cohorts with and without dexamethasone (40 mg/week). Patients in the dose-escalation (N = 31) and Phase 2 single-agent (N = 32) cohorts had received prior bortezomib as well as prior thalidomide and/or lenalidomide. Patients in the Phase 2 Filanesib plus dexamethasone cohort (N = 55) had received prior alkylator therapy and had disease refractory to lenalidomide, bortezomib, and dexamethasone. Prophylactic filgrastim was incorporated during dose escalation and was used throughout Phase 2.
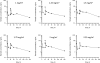
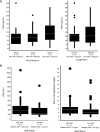
Results: Patients in each cohort had received a median of ≥6 prior therapies. The most common dose-limiting toxicities were febrile neutropenia and mucosal inflammation. In Phase 2, Grade 3 and 4 cytopenias were reported in approximately 50% of patients. Nonhematologic toxicities were infrequent. Phase 2 response rates (partial responses or better) were 16% (single agent) and 15% (Filanesib plus dexamethasone). All responding patients had low baseline levels of α1-acid glycoprotein, a potential selective biomarker.
Conclusions: Filanesib 1.50 mg/m2 /day administered with prophylactic filgrastim has a manageable safety profile and encouraging activity in heavily pretreated patients This study is registered at www.clinicaltrials.gov as NCT00821249. Cancer 2017;123:4617-4630. © 2017 American Cancer Society.
Keywords: dexamethasone; filanesib. kinesin; maximum tolerated dose; multiple myeloma; pharmacokinetics; spindle poles.
© 2017 American Cancer Society.
Figures


References
-
- Blangy A, Lane HA, d'Hérin P, et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83(7):1159–1169. - PubMed
-
- Stern BM, Murray AW. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr Biol. 2001;11(18):1462–1467. - PubMed
-
- Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88(18):1308–1314. - PubMed
-
- Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther. 2010;9(7):2046–2056. - PubMed
-
- Woessner RD, Corrette C, Allen S, et al. ARRY-520, a KSP inhibitor with efficacy and pharmacodynamic activity in animal models of solid tumors. Cancer Res. 2007;67:1433.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

