Cutaneous Eruptions in Patients Receiving Immune Checkpoint Blockade: Clinicopathologic Analysis of the Nonlichenoid Histologic Pattern
- PMID: 28817405
- PMCID: PMC5657299
- DOI: 10.1097/PAS.0000000000000900
Cutaneous Eruptions in Patients Receiving Immune Checkpoint Blockade: Clinicopathologic Analysis of the Nonlichenoid Histologic Pattern
Abstract
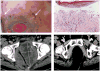
Cutaneous eruptions are among the most common immune-related adverse events (irAEs) associated with anti-programmed cell death protein 1/programmed cell death ligand 1 therapy, and are often clinically and histologically characterized as lichenoid. Nonlichenoid patterns may also occur and are likely to be encountered by surgical pathologists, given the increasing clinical use of these agents. The purpose of this study is to describe the histopathologic features of nonlichenoid cutaneous irAEs from patients receiving anti-programmed cell death protein 1/programmed cell death ligand 1 therapies for a variety of underlying advanced malignancies. Sixteen patients with 17 biopsied eruptions were included from 2 academic institutions with extensive experience administering and monitoring responses to immune checkpoint blockade as well as treating the potential side effects. Eruptions occurred a median of 10 days (range, 1 d to 11.4 mo) after treatment initiation. Nearly half of specimens demonstrated either a psoriasiform/spongiotic or an urticarial-type reaction pattern on histologic review. Patterns consistent with Grover disease, bullous pemphigoid, and granulomatous dermatitis were also observed. Nearly two-thirds of patients required systemic corticosteroids for treatment of the cutaneous irAE, and 19% of patients discontinued immunotherapy due to their skin eruptions. 75% of patients showed an objective antitumor response. The diverse array of nonlichenoid cutaneous irAE presented here should reflect and inform the scope of histologic patterns encountered by the practicing surgical pathologist. Such eruptions are seen in patients with a variety of underlying tumor types, many of whom ultimately demonstrate a favorable response to immune checkpoint blockade.
Conflict of interest statement
Figures




References
-
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

