Comparing efficacies of moxifloxacin, levofloxacin and gatifloxacin in tuberculosis granulomas using a multi-scale systems pharmacology approach
- PMID: 28817561
- PMCID: PMC5560534
- DOI: 10.1371/journal.pcbi.1005650
Comparing efficacies of moxifloxacin, levofloxacin and gatifloxacin in tuberculosis granulomas using a multi-scale systems pharmacology approach
Abstract
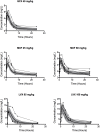
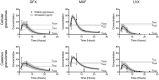
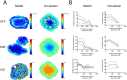
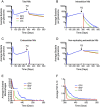
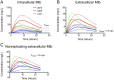
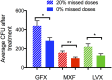
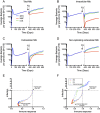
Granulomas are complex lung lesions that are the hallmark of tuberculosis (TB). Understanding antibiotic dynamics within lung granulomas will be vital to improving and shortening the long course of TB treatment. Three fluoroquinolones (FQs) are commonly prescribed as part of multi-drug resistant TB therapy: moxifloxacin (MXF), levofloxacin (LVX) or gatifloxacin (GFX). To date, insufficient data are available to support selection of one FQ over another, or to show that these drugs are clinically equivalent. To predict the efficacy of MXF, LVX and GFX at a single granuloma level, we integrate computational modeling with experimental datasets into a single mechanistic framework, GranSim. GranSim is a hybrid agent-based computational model that simulates granuloma formation and function, FQ plasma and tissue pharmacokinetics and pharmacodynamics and is based on extensive in vitro and in vivo data. We treat in silico granulomas with recommended daily doses of each FQ and compare efficacy by multiple metrics: bacterial load, sterilization rates, early bactericidal activity and efficacy under non-compliance and treatment interruption. GranSim reproduces in vivo plasma pharmacokinetics, spatial and temporal tissue pharmacokinetics and in vitro pharmacodynamics of these FQs. We predict that MXF kills intracellular bacteria more quickly than LVX and GFX due in part to a higher cellular accumulation ratio. We also show that all three FQs struggle to sterilize non-replicating bacteria residing in caseum. This is due to modest drug concentrations inside caseum and high inhibitory concentrations for this bacterial subpopulation. MXF and LVX have higher granuloma sterilization rates compared to GFX; and MXF performs better in a simulated non-compliance or treatment interruption scenario. We conclude that MXF has a small but potentially clinically significant advantage over LVX, as well as LVX over GFX. We illustrate how a systems pharmacology approach combining experimental and computational methods can guide antibiotic selection for TB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- WHO, Global tuberculosis report. 2015.
-
- Lin P.L., et al., Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun, 2009. 77(10): p. 4631–42. doi: 10.1128/IAI.00592-09 - DOI - PMC - PubMed
-
- Ramakrishnan L., Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol, 2012. 12(5): p. 352–66. doi: 10.1038/nri3211 - DOI - PubMed
-
- Lenaerts A., Barry C.E. 3rd, and Dartois V., Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev, 2015. 264(1): p. 288–307. doi: 10.1111/imr.12252 - DOI - PMC - PubMed
-
- WHO, WHO treatment guidelines for drug-resistant tuberculosis. 2016: Geneva, Switzerland. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

