Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits
- PMID: 28817803
- PMCID: PMC5595250
- DOI: 10.1016/j.neuron.2017.07.034
Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits
Abstract
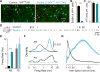
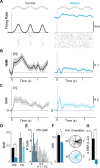
GABAergic interneurons play important roles in cortical circuit development. However, there are multiple populations of interneurons and their respective developmental contributions remain poorly explored. Neuregulin 1 (NRG1) and its interneuron-specific receptor ERBB4 are critical genes for interneuron maturation. Using a conditional ErbB4 deletion, we tested the role of vasoactive intestinal peptide (VIP)-expressing interneurons in the postnatal maturation of cortical circuits in vivo. ErbB4 removal from VIP interneurons during development leads to changes in their activity, along with severe dysregulation of cortical temporal organization and state dependence. These alterations emerge during adolescence, and mature animals in which VIP interneurons lack ErbB4 exhibit reduced cortical responses to sensory stimuli and impaired sensory learning. Our data support a key role for VIP interneurons in cortical circuit development and suggest a possible contribution to pathophysiology in neurodevelopmental disorders. These findings provide a new perspective on the role of GABAergic interneuron diversity in cortical development. VIDEO ABSTRACT.
Keywords: ErbB4; GABAergic; VIP; cholinergic; cortex; development; gCAMP6; interneuron; somatostatin; visual.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


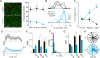


References
-
- Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:5699–5709. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

