β-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy
- PMID: 28819022
- PMCID: PMC5645237
- DOI: 10.1158/0008-5472.CAN-17-0546
β-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy
Abstract
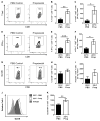
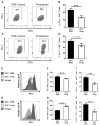
The immune context of tumors has significant prognostic value and is predictive of responsiveness to several forms of therapy, including immunotherapy. We report here that CD8+ T-cell frequency and functional orientation within the tumor microenvironment is regulated by β2-adrenergic receptor (β-AR) signaling in host immune cells. We used three strategies-physiologic (manipulation of ambient thermal environment), pharmacologic (β-blockers), and genetic (β2-AR knockout mice) to reduce adrenergic stress signaling in two widely studied preclinical mouse tumor models. Reducing β-AR signaling facilitated conversion of tumors to an immunologically active tumor microenvironment with increased intratumoral frequency of CD8+ T cells with an effector phenotype and decreased expression of programmed death receptor-1 (PD-1), in addition to an elevated effector CD8+ T-cell to CD4+ regulatory T-cell ratio (IFNγ+CD8+:Treg). Moreover, this conversion significantly increased the efficacy of anti-PD-1 checkpoint blockade. These data highlight the potential of adrenergic stress and norepinephrine-driven β-AR signaling to regulate the immune status of the tumor microenvironment and support the strategic use of clinically available β-blockers in patients to improve responses to immunotherapy. Cancer Res; 77(20); 5639-51. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



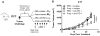

Similar articles
-
Chronic Adrenergic Stress Contributes to Metabolic Dysfunction and an Exhausted Phenotype in T Cells in the Tumor Microenvironment.Cancer Immunol Res. 2021 Jun;9(6):651-664. doi: 10.1158/2326-6066.CIR-20-0445. Epub 2021 Mar 24. Cancer Immunol Res. 2021. PMID: 33762351 Free PMC article.
-
Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through β2-Adrenergic Receptor Signaling.J Immunol. 2015 Nov 15;195(10):5045-54. doi: 10.4049/jimmunol.1500700. Epub 2015 Oct 12. J Immunol. 2015. PMID: 26459348 Free PMC article.
-
β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress.Cancer Immunol Immunother. 2019 Jan;68(1):11-22. doi: 10.1007/s00262-018-2243-8. Epub 2018 Sep 18. Cancer Immunol Immunother. 2019. PMID: 30229289 Free PMC article.
-
Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer.J Gynecol Oncol. 2017 Sep;28(5):e64. doi: 10.3802/jgo.2017.28.e64. Epub 2017 Jun 5. J Gynecol Oncol. 2017. PMID: 28657225 Free PMC article. Review.
-
Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response.Front Immunol. 2018 Feb 6;9:164. doi: 10.3389/fimmu.2018.00164. eCollection 2018. Front Immunol. 2018. PMID: 29479349 Free PMC article. Review.
Cited by
-
Clinical Use of Propranolol Reduces Biomarkers of Proliferation in Gastric Cancer.Front Oncol. 2021 Apr 26;11:628613. doi: 10.3389/fonc.2021.628613. eCollection 2021. Front Oncol. 2021. PMID: 33981600 Free PMC article.
-
The Role of Neural Signaling in the Pancreatic Cancer Microenvironment.Cancers (Basel). 2022 Aug 31;14(17):4269. doi: 10.3390/cancers14174269. Cancers (Basel). 2022. PMID: 36077804 Free PMC article. Review.
-
Autonomic modulation of the immune response and implications for CNS malignancies.NPJ Precis Oncol. 2025 Jun 7;9(1):168. doi: 10.1038/s41698-025-00957-y. NPJ Precis Oncol. 2025. PMID: 40483275 Free PMC article. Review.
-
Using Mice to Model Human Disease: Understanding the Roles of Baseline Housing-Induced and Experimentally Imposed Stresses in Animal Welfare and Experimental Reproducibility.Animals (Basel). 2022 Feb 3;12(3):371. doi: 10.3390/ani12030371. Animals (Basel). 2022. PMID: 35158694 Free PMC article. Review.
-
Neuro-immune cross-talk in cancer.Nat Rev Cancer. 2025 Aug;25(8):573-589. doi: 10.1038/s41568-025-00831-w. Epub 2025 Jun 16. Nat Rev Cancer. 2025. PMID: 40523971 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

