Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells
- PMID: 28819024
- PMCID: PMC5626640
- DOI: 10.1158/0008-5472.CAN-16-2774
Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells
Abstract
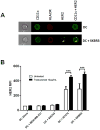
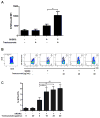
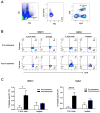
Early-phase clinical trials evaluating CD8+ T cell-eliciting, HER2-derived peptide vaccines administered to HER2+ breast cancer patients in the adjuvant setting suggest synergy between the vaccines and trastuzumab, the mAb targeting the HER2 protein. Among 60 patients enrolled in clinical trials evaluating the E75 + GM-CSF and GP2 + GM-CSF vaccines, there have been no recurrences in patients vaccinated after receiving trastuzumab as part of standard therapy in the per treatment analyses conducted after a median follow-up of greater than 34 months. Here, we describe a mechanism by which this synergy may occur. Flow cytometry showed that trastuzumab facilitated uptake of HER2 by dendritic cells (DC), which was mediated by the Fc receptor and was specific to trastuzumab. In vitro, increased HER2 uptake by DC increased cross-presentation of E75, the immunodominant epitope derived from the HER2 protein, an observation confirmed in two in vivo mouse models. This increased E75 cross-presentation, mediated by trastuzumab treatment, enabled more efficient expansion of E75-specific cytotoxic T cells (E75-CTL). These results demonstrate a mechanism by which trastuzumab links innate and adaptive immunity by facilitating activation of antigen-specific T cells. On the basis of these data, we conclude that HER2-positive breast cancer patients that have been treated with trastuzumab may experience a more robust antitumor immune response by restimulation of T cells with the E75 peptide vaccine, thereby accounting for the improved disease-free survival observed with combination therapy. Cancer Res; 77(19); 5374-83. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

