The Tumor Microenvironment Regulates Sensitivity of Murine Lung Tumors to PD-1/PD-L1 Antibody Blockade
- PMID: 28819064
- PMCID: PMC5787226
- DOI: 10.1158/2326-6066.CIR-16-0365
The Tumor Microenvironment Regulates Sensitivity of Murine Lung Tumors to PD-1/PD-L1 Antibody Blockade
Abstract
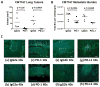
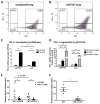
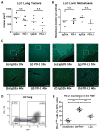
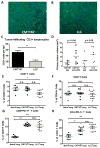
Immune checkpoint inhibitors targeting the interaction between programmed cell death-1 (PD-1) and its ligand PD-L1 induce tumor regression in a subset of non-small cell lung cancer patients. However, clinical response rates are less than 25%. Evaluation of combinations of immunotherapy with existing therapies requires appropriate preclinical animal models. In this study, murine lung cancer cells (CMT167 and LLC) were implanted either orthotopically in the lung or subcutaneously in syngeneic mice, and response to anti-PD-1/PD-L1 therapy was determined. Anti-PD-1/PD-L1 therapy inhibited CMT167 orthotopic lung tumors by 95%. The same treatments inhibited CMT167 subcutaneous tumors by only 30% and LLC orthotopic lung tumors by 35%. CMT167 subcutaneous tumors had more Foxp3+ CD4+ T cells and fewer PD-1+ CD4+ T cells compared with CMT167 orthotopic tumors. Flow cytometric analysis also demonstrated increased abundance of PD-L1high cells in the tumor microenvironment in CMT167 tumor-bearing lungs compared with CMT167 subcutaneous tumors or LLC tumor-bearing lungs. Silencing PD-L1 expression in CMT167 cells resulted in smaller orthotopic tumors that remained sensitive to anti-PD-L1 therapy, whereas implantation of CMT167 cells into PD-L1- mice blocked orthotopic tumor growth, indicating a role for PD-L1 in both the cancer cell and the microenvironment. These findings indicate that the response of cancer cells to immunotherapy will be determined by both intrinsic properties of the cancer cells and specific interactions with the microenvironment. Experimental models that accurately recapitulate the lung tumor microenvironment are useful for evaluation of immunotherapeutic agents. Cancer Immunol Res; 5(9); 767-77. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures

References
-
- Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Current topics in microbiology and immunology. 2011;350:17–37. - PubMed
-
- Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(18):2004–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

