Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau
- PMID: 28819146
- PMCID: PMC5561136
- DOI: 10.1038/s41467-017-00480-0
Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau
Abstract
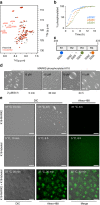
The protein Tau aggregates into tangles in the brain of patients with Alzheimer's disease. In solution, however, Tau is intrinsically disordered, highly soluble, and binds to microtubules. It is still unclear what initiates the conversion from an innocuous phase of high solubility and functionality to solid-like neurotoxic deposits. Here, we show that the microtubule-binding repeats of Tau, which are lysine-rich, undergo liquid-liquid phase separation in solution. Liquid-liquid demixing causes molecular crowding of amyloid-promoting elements of Tau and drives electrostatic coacervation. Furthermore, we demonstrate that three-repeat and four-repeat isoforms of Tau differ in their ability for demixing. Alternative splicing of Tau can thus regulate the formation of Tau-containing membrane-less compartments. In addition, phosphorylation of Tau repeats promotes liquid-liquid phase separation at cellular protein conditions. The combined data propose a mechanism in which liquid droplets formed by the positively charged microtubule-binding domain of Tau undergo coacervation with negatively charged molecules to promote amyloid formation.Tau forms aggregates in the brains of Alzheimer patients. Here, the authors identify conditions, where the microtubule-binding repeats of Tau undergo a phosphorylation-dependent liquid-liquid phase separation, leading to molecular crowding in the formed Tau liquid droplets and characterize them by NMR and other biophysical methods.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

