Deletion of EP4 in S100a4-lineage cells reduces scar tissue formation during early but not later stages of tendon healing
- PMID: 28819185
- PMCID: PMC5561254
- DOI: 10.1038/s41598-017-09407-7
Deletion of EP4 in S100a4-lineage cells reduces scar tissue formation during early but not later stages of tendon healing
Abstract
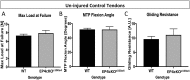
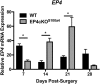
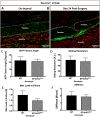
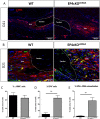
Tendon injuries heal via scar tissue rather than regeneration. This healing response forms adhesions between the flexor tendons in the hand and surrounding tissues, resulting in impaired range of motion and hand function. Mechanistically, inflammation has been strongly linked to adhesion formation, and Prostaglandin E2 (PGE2) is associated with both adhesion formation and tendinopathy. In the present study we tested the hypothesis that deletion of the PGE2 receptor EP4 in S100a4-lineage cells would decrease adhesion formation. S100a4-Cre; EP4 flox/flox (EP4cKOS100a4) repairs healed with improved gliding function at day 14, followed by impaired gliding at day 28, relative to wild type. Interestingly, EP4cKOS100a4 resulted in only transient deletion of EP4, suggesting up-regulation of EP4 in an alternative cell population in these mice. Loss of EP4 in Scleraxis-lineage cells did not alter gliding function, suggesting that Scx-lineage cells are not the predominant EP4 expressing population. In contrast, a dramatic increase in α-SMA+, EP4+ double-positive cells were observed in EP4cKOS100a4 suggesting that EP4cKOS100a4 repairs heal with increased infiltration of EP4 expressing α-SMA myofibroblasts, identifying a potential mechanism of late up-regulation of EP4 and impaired gliding function in EP4cKOS100a4 tendon repairs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

