FBN30 in wild Anopheles gambiae functions as a pathogen recognition molecule against clinically circulating Plasmodium falciparum in malaria endemic areas in Kenya
- PMID: 28819256
- PMCID: PMC5561218
- DOI: 10.1038/s41598-017-09017-3
FBN30 in wild Anopheles gambiae functions as a pathogen recognition molecule against clinically circulating Plasmodium falciparum in malaria endemic areas in Kenya
Abstract
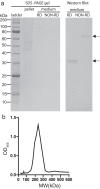
Malaria is a worldwide health problem that affects two-thirds of the world population. Plasmodium invasion of anopheline mosquitoes is an obligatory step for malaria transmission. However, mosquito-malaria molecular interactions in nature are not clear. A genetic variation within mosquito fibrinogen related-protein 30 (FBN30) was previously identified to be associated with Plasmodium falciparum infection in natural Anopheles gambiae populations at malaria endemic areas in Kenya, and reducing FBN30 expression by RNAi makes mosquitoes more susceptible to P. berghei. New results show that FBN30 is a secreted octamer that binds to both P. berghei and clinically circulating P. falciparum from malaria endemic areas in Kenya, but not laboratory P. falciparum strain NF54. Moreover, the natural genetic mutation (T to C) within FBN30 signal peptide, which changes the position 10 amino acid from phenylalanine to leucine, reduces protein expression by approximately half. This change is consistent to more susceptible An. gambiae to P. falciparum infection in the field. FBN30 in natural An. gambiae is proposed to work as a pathogen recognition molecule in inhibiting P. falciparum transmission in malaria endemic areas.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

