Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3
- PMID: 28819304
- PMCID: PMC5561029
- DOI: 10.1038/s41598-017-08581-y
Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3
Abstract
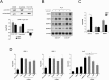
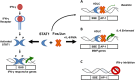
Dynamic remodelling of the extracellular matrix (ECM) is a key feature of cancer progression. Enzymes that modify the ECM, such as matrix metalloproteinases (MMPs), have long been recognised as important targets of anticancer therapy. Inflammatory cytokines are known to play a key role in regulating protease expression in cancer. Here we describe the identification of gamma-activated site (GAS)-like, signal transducer and activator of transcription (STAT) binding elements (SBEs) within the proximal promoters of the MMP-1 and MMP-3 genes, which in association with AP-1 components (c-Fos or Jun), bind STAT-1 in a homodimer like complex (HDLC). We further demonstrate that MMP expression and binding of this complex to SBEs can either be enhanced by interleukin (IL)-6, or reduced by interferon gamma (IFN-γ), and that IL-6 regulation of MMPs is not STAT-3 dependent. Collectively, this data adds to existing understanding of the mechanism underlying cytokine regulation of MMP expression via STAT-1, and increases our understanding of the links between inflammation and malignancy in colon cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

