Technique of retinal gene therapy: delivery of viral vector into the subretinal space
- PMID: 28820183
- PMCID: PMC5601444
- DOI: 10.1038/eye.2017.158
Technique of retinal gene therapy: delivery of viral vector into the subretinal space
Abstract
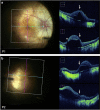
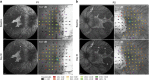
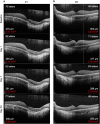
PurposeSafe and reproducible delivery of gene therapy vector into the subretinal space is essential for successful targeting of the retinal pigment epithelium (RPE) and photoreceptors. The success of surgery is critical for the clinical efficacy of retinal gene therapy. Iatrogenic detachment of the degenerate (often adherent) retina in patients with hereditary retinal degenerations and small volume (eg, 0.1 ml) subretinal injections pose new surgical challenges.MethodsOur subretinal gene therapy technique involved pre-operative planning with optical coherence tomography (OCT) and autofluorescence (AF) imaging, 23 G pars plana vitrectomy, internal limiting membrane staining with Membrane Blue Dual (DORC BV, Zuidland, Netherlands), a two-step subretinal injection using a 41 G Teflon tipped cannula (DORC) first with normal saline to create a parafoveal bleb followed by slow infusion of viral vector via the same self-sealing retinotomy. Surgical precision was further enhanced by intraoperative OCT (Zeiss Rescan 7000, Carl Zeiss Meditec AG, Jena, Germany). Foveal functional and structural recovery was evaluated using best-corrected Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, microperimetry and OCT.ResultsTwo patients with choroideremia aged 29 (P1) and 27 (P2) years, who had normal and symmetrical levels of best-corrected visual acuity (BCVA) in both eyes, underwent unilateral gene therapy with the fellow eye acting as internal control. The surgeries were uncomplicated in both cases with successful detachment of the macula by subretinal vector injection. Both treated eyes showed recovery of BCVA (P1: 76-77 letters; P2: 84-88 letters) and mean threshold sensitivity of the central macula (P1: 10.7-10.7 dB; P2: 14.2-14.1 dB) to baseline within a month. This was accompanied by normalisation of central retinal thickness on OCT.ConclusionsHerein we describe a reliable technique for subretinal gene therapy, which is currently used in clinical trials to treat choroideremia using an adeno-associated viral (AAV) vector encoding the CHM gene. Strategies to minimise potential complications, such as avoidance of excessive retinal stretch, air bubbles within the injection system, reflux of viral vector and post-operative vitritis are discussed.
Conflict of interest statement
REM: a founding director of NightstaRx (London, UK)—a gene therapy company established by the University of Oxford and funded by the Wellcome Trust. REM is a named inventor on a patent ‘Vector administration and dosing’ filed on behalf of the University of Oxford (US Patent Application No. 14/598,948). The remaining authors declare no conflict of interest.
Figures


References
-
- Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 2016; 388: 661–672. - PMC - PubMed
-
- Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet 2016; 135: 327–343. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

