Discovery of a Potent Inhibitor Class with High Selectivity toward Clostridial Collagenases
- PMID: 28820255
- PMCID: PMC5607459
- DOI: 10.1021/jacs.7b06935
Discovery of a Potent Inhibitor Class with High Selectivity toward Clostridial Collagenases
Abstract
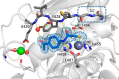
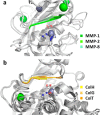
Secreted virulence factors like bacterial collagenases are conceptually attractive targets for fighting microbial infections. However, previous attempts to develop potent compounds against these metalloproteases failed to achieve selectivity against human matrix metalloproteinases (MMPs). Using a surface plasmon resonance-based screening complemented with enzyme inhibition assays, we discovered an N-aryl mercaptoacetamide-based inhibitor scaffold that showed sub-micromolar affinities toward collagenase H (ColH) from the human pathogen Clostridium histolyticum. Moreover, these inhibitors also efficiently blocked the homologous bacterial collagenases, ColG from C. histolyticum, ColT from C. tetani, and ColQ1 from the Bacillus cereus strain Q1, while showing negligible activity toward human MMPs-1, -2, -3, -7, -8, and -14. The most active compound displayed a more than 1000-fold selectivity over human MMPs. This selectivity can be rationalized by the crystal structure of ColH with this compound, revealing a distinct non-primed binding mode to the active site. The non-primed binding mode presented here paves the way for the development of selective broad-spectrum bacterial collagenase inhibitors with potential therapeutic application in humans.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Cato E.; George W.; Finegold S.. Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Baltimore, 1986; pp 1141–1200.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

