BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2
- PMID: 28820284
- PMCID: PMC5640199
- DOI: 10.1080/15548627.2017.1357792
BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2
Abstract
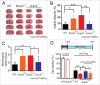
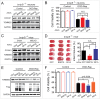
Cerebral ischemia induces massive mitochondrial damage. These damaged mitochondria are cleared, thus attenuating brain injury, by mitophagy. Here, we identified the involvement of BNIP3L/NIX in cerebral ischemia-reperfusion (I-R)-induced mitophagy. Bnip3l knockout (bnip3l-/-) impaired mitophagy and aggravated cerebral I-R injury in mice, which can be rescued by BNIP3L overexpression. The rescuing effects of BNIP3L overexpression can be observed in park2-/- mice, which showed mitophagy deficiency after I-R. Interestingly, bnip3l and park2 double-knockout mice showed a synergistic mitophagy deficiency with I-R treatment, which further highlighted the roles of BNIP3L-mediated mitophagy as being independent from PARK2. Further experiments indicated that phosphorylation of BNIP3L serine 81 is critical for BNIP3L-mediated mitophagy. Nonphosphorylatable mutant BNIP3LS81A failed to counteract both mitophagy impairment and neuroprotective effects in bnip3l-/- mice. Our findings offer insights into mitochondrial quality control in ischemic stroke and bring forth the concept that BNIP3L could be a potential therapeutic target for ischemic stroke, beyond its accepted role in reticulocyte maturation.
Keywords: BNIP3L/NIX; PARK2/PARKIN; cerebral ischemia; mitophagy; phosphorylation.
Figures





References
-
- Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, et al.. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 2014;10:1801-13. doi:10.4161/auto.32136. PMID:25126734 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
