Determination of Sphingosine-1-Phosphate in Human Plasma Using Liquid Chromatography Coupled with Q-Tof Mass Spectrometry
- PMID: 28820460
- PMCID: PMC5578187
- DOI: 10.3390/ijms18081800
Determination of Sphingosine-1-Phosphate in Human Plasma Using Liquid Chromatography Coupled with Q-Tof Mass Spectrometry
Abstract
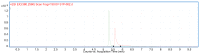
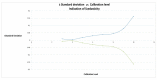
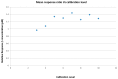
Evidence suggests that high-density lipoprotein (HDL) components distinct from cholesterol, such as sphingosine-1-phosphate (S1P), may account for the anti-atherothrombotic effects attributed to this lipoprotein. The current method for the determination of plasma levels of S1P as well as levels associated with HDL particles is still cumbersome an assay method to be worldwide practical. Recently, a simplified protocol based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the sensitive and specific quantification of plasma levels of S1P with good accuracy has been reported. This work utilized a triple quadrupole (QqQ)-based LC-MS/MS system. Here we adapt that method for the determination of plasma levels of S1P using a quadrupole time of flight (Q-Tof) based LC-MS system. Calibration curves were linear in the range of 0.05 to 2 µM. The lower limit of quantification (LOQ) was 0.05 µM. The concentration of S1P in human plasma was determined to be 1 ± 0.09 µM (n = 6). The average accuracy over the stated range of the method was found to be 100 ± 5.9% with precision at the LOQ better than 10% when predicting the calibration standards. The concentration of plasma S1P in the prepared samples was stable for 24 h at room temperature. We have demonstrated the quantification of plasma S1P using Q-Tof based LC-MS with very good sensitivity, accuracy, and precision that can used for future studies in this field.
Keywords: high-density lipoprotein; high-density lipoprotein (HDL); liquid chromatography-mass spectrometry (LC-MS); liquid chromatography-tandem mass spectrometry (LC-MS/MS); quadrupole time of flight (Q-Tof); sphingosine-1-phosphate (S1P).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- De Backer G., Ambrosionie E., Borch-Johnsen K., Brotons C., Cifkova R., Dallongeville J., Ebrahim S., Faergeman O., Graham I., Mancia G. European guidelines on cardiovascular disease prevention in clinical practice: Third joint task force of european and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts) Eur. J. Cardiovasc. Prev. Rehabil. 2003;10:S1–S10. - PubMed
-
- World Health Organization Cardiovascular Diseases (cvds). Fact Sheet n 317. [(accessed on 5 November 2012)];2011 Sep; Available online: http://www.whoint/mediacentre/factsheets/fs317/en/index html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

