A Third Generation Glucose Biosensor Based on Cellobiose Dehydrogenase Immobilized on a Glassy Carbon Electrode Decorated with Electrodeposited Gold Nanoparticles: Characterization and Application in Human Saliva
- PMID: 28820469
- PMCID: PMC5579551
- DOI: 10.3390/s17081912
A Third Generation Glucose Biosensor Based on Cellobiose Dehydrogenase Immobilized on a Glassy Carbon Electrode Decorated with Electrodeposited Gold Nanoparticles: Characterization and Application in Human Saliva
Abstract
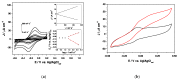
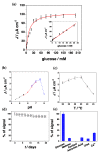
Efficient direct electron transfer (DET) between a cellobiose dehydrogenase mutant from Corynascus thermophilus (CtCDH C291Y) and a novel glassy carbon (GC)-modified electrode, obtained by direct electrodeposition of gold nanoparticles (AuNPs) was realized. The electrode was further modified with a mixed self-assembled monolayer of 4-aminothiophenol (4-APh) and 4-mercaptobenzoic acid (4-MBA), by using glutaraldehyde (GA) as cross-linking agent. The CtCDH C291Y/GA/4-APh,4-MBA/AuNPs/GC platform showed an apparent heterogeneous electron transfer rate constant (ks) of 19.4 ± 0.6 s-1, with an enhanced theoretical and real enzyme surface coverage (Γtheor and Γreal) of 5287 ± 152 pmol cm-2 and 27 ± 2 pmol cm-2, respectively. The modified electrode was successively used as glucose biosensor exhibiting a detection limit of 6.2 μM, an extended linear range from 0.02 to 30 mM, a sensitivity of 3.1 ± 0.1 μA mM-1 cm-2 (R2 = 0.995), excellent stability and good selectivity. These performances compared favourably with other glucose biosensors reported in the literature. Finally, the biosensor was tested to quantify the glucose content in human saliva samples with successful results in terms of both recovery and correlation with glucose blood levels, allowing further considerations on the development of non-invasive glucose monitoring devices.
Keywords: cellobiose dehydrogenase; electrodeposition; glucose biosensor; gold nanoparticles; human saliv.
Conflict of interest statement
The authors declare no conflicts of interest. All authors have revised and approved the final version.
Figures




References
-
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

