National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer
- PMID: 28820644
- PMCID: PMC5707208
- DOI: 10.1200/JCO.2017.73.6314
National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer
Erratum in
-
Errata.J Clin Oncol. 2018 Feb 1;36(4):432. doi: 10.1200/JCO.2017.77.5114. J Clin Oncol. 2018. PMID: 29378158 Free PMC article. No abstract available.
Abstract
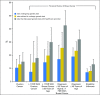
Purpose In the United States, 3.8 million women have a history of breast (BC) or ovarian cancer (OC). Up to 15% of cases are attributable to heritable mutations, which, if identified, provide critical knowledge for treatment and preventive care. It is unknown how many patients who are at high risk for these mutations have not been tested and how rates vary by risk criteria. Methods We used pooled cross-sectional data from three Cancer Control Modules (2005, 2010, 2015) of the National Health Interview Survey, a national in-person household interview survey. Eligible patients were adult females with a history of BC and/or OC meeting select 2017 National Comprehensive Cancer Network eligibility criteria on the basis of age of diagnosis and family history. Outcomes included the proportion of individuals reporting a history of discussing genetic testing with a health professional, being advised to undergo genetic testing, or undergoing genetic testing for BC or OC. Results Of 47,218 women, 2.7% had a BC history and 0.4% had an OC history. For BC, 35.6% met one or more select eligibility criteria; of those, 29.0% discussed, 20.2% were advised to undergo, and 15.3% underwent genetic testing. Testing rates for individual eligibility criteria ranged from 6.2% (relative with OC) to 18.2% (diagnosis ≤ 45 years of age). For OC, 15.1% discussed, 13.1% were advised to undergo, and 10.5% underwent testing. Using only four BC eligibility criteria and all patients with OC, an estimated 1.2 to 1.3 million individuals failed to receive testing. Conclusion Fewer than one in five individuals with a history of BC or OC meeting select National Cancer Comprehensive Network criteria have undergone genetic testing. Most have never discussed testing with a health care provider. Large national efforts are warranted to address this unmet need.
Figures
Comment in
-
Genetic Testing: What Problem Are We Trying to Solve?J Clin Oncol. 2017 Dec 1;35(34):3789-3791. doi: 10.1200/JCO.2017.74.7899. Epub 2017 Aug 18. J Clin Oncol. 2017. PMID: 28820645 No abstract available.
-
Adaptation of Genetic Counseling According to an Individual's Literacy Regarding Genomics.J Clin Oncol. 2018 Feb 10;36(5):516-517. doi: 10.1200/JCO.2017.76.3300. Epub 2018 Jan 2. J Clin Oncol. 2018. PMID: 29293385 No abstract available.
-
Reply to S. Nakamura et al and S. Narod et al.J Clin Oncol. 2018 Feb 10;36(5):517-518. doi: 10.1200/JCO.2017.76.4100. Epub 2018 Jan 2. J Clin Oncol. 2018. PMID: 29293387 No abstract available.
-
Population-Based Genetic Testing for BRCA1 and BRCA2.J Clin Oncol. 2018 Feb 10;36(5):517. doi: 10.1200/JCO.2017.75.8490. Epub 2018 Jan 2. J Clin Oncol. 2018. PMID: 29293388 No abstract available.
References
-
- American Cancer Society: Breast cancer facts & figures 2015-2016. Atlanta, GA, American Cancer Society, 2015 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-....
-
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2017. CA Cancer J Clin 67:7-30, 2017 - PubMed
-
- Byrski T, Huzarski T, Dent R, et al. : Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 115:359-363, 2009 - PubMed
-
- Fong PC, Boss DS, Yap TA, et al. : Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123-134, 2009 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous