Src Kinase Inhibition Attenuates Morphine Tolerance without Affecting Reinforcement or Psychomotor Stimulation
- PMID: 28820778
- PMCID: PMC5648044
- DOI: 10.1097/ALN.0000000000001834
Src Kinase Inhibition Attenuates Morphine Tolerance without Affecting Reinforcement or Psychomotor Stimulation
Abstract
Background: Prolonged opioid administration leads to tolerance characterized by reduced analgesic potency. Pain management is additionally compromised by the hedonic effects of opioids, the cause of their misuse. The multifunctional protein β-arrestin2 regulates the hedonic effects of morphine and participates in tolerance. These actions might reflect µ opioid receptor up-regulation through reduced endocytosis. β-Arrestin2 also recruits kinases to µ receptors. We explored the role of Src kinase in morphine analgesic tolerance, locomotor stimulation, and reinforcement in C57BL/6 mice.
Methods: Analgesic (tail withdrawal latency; percentage of maximum possible effect, n = 8 to 16), locomotor (distance traveled, n = 7 to 8), and reinforcing (conditioned place preference, n = 7 to 8) effects of morphine were compared in wild-type, µ, µ, and β-arrestin2 mice. The influence of c-Src inhibitors dasatinib (n = 8) and PP2 (n = 12) was examined.
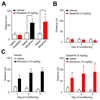
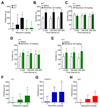
Results: Analgesia in morphine-treated wild-type mice exhibited tolerance, declining by day 10 to a median of 62% maximum possible effect (interquartile range, 29 to 92%). Tolerance was absent from mice receiving dasatinib. Tolerance was enhanced in µ mice (34% maximum possible effect; interquartile range, 5 to 52% on day 5); dasatinib attenuated tolerance (100% maximum possible effect; interquartile range, 68 to 100%), as did PP2 (91% maximum possible effect; interquartile range, 78 to 100%). By contrast, c-Src inhibition affected neither morphine-evoked locomotor stimulation nor reinforcement. Remarkably, dasatinib not only attenuated tolerance but also reversed established tolerance in µ mice.
Conclusions: The ability of c-Src inhibitors to inhibit tolerance, thereby restoring analgesia, without altering the hedonic effect of morphine, makes c-Src inhibitors promising candidates as adjuncts to opioid analgesics.
Conflict of interest statement
Figures




References
-
- Webster LR, Fine PG. Review and critique of opioid rotation practices and associated risks of toxicity. Pain Med. 2012;13:562–70. - PubMed
-
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

