Systematic Identification of MCU Modulators by Orthogonal Interspecies Chemical Screening
- PMID: 28820965
- PMCID: PMC5825229
- DOI: 10.1016/j.molcel.2017.07.019
Systematic Identification of MCU Modulators by Orthogonal Interspecies Chemical Screening
Abstract
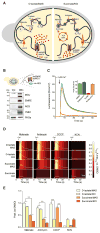
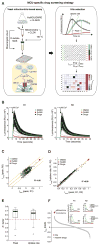
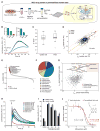
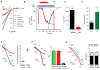
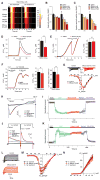
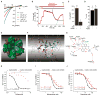
The mitochondrial calcium uniporter complex is essential for calcium (Ca2+) uptake into mitochondria of all mammalian tissues, where it regulates bioenergetics, cell death, and Ca2+ signal transduction. Despite its involvement in several human diseases, we currently lack pharmacological agents for targeting uniporter activity. Here we introduce a high-throughput assay that selects for human MCU-specific small-molecule modulators in primary drug screens. Using isolated yeast mitochondria, reconstituted with human MCU, its essential regulator EMRE, and aequorin, and exploiting a D-lactate- and mannitol/sucrose-based bioenergetic shunt that greatly minimizes false-positive hits, we identify mitoxantrone out of more than 600 clinically approved drugs as a direct selective inhibitor of human MCU. We validate mitoxantrone in orthogonal mammalian cell-based assays, demonstrating that our screening approach is an effective and robust tool for MCU-specific drug discovery and, more generally, for the identification of compounds that target mitochondrial functions.
Keywords: MCU; bioenergetics; calcium; calcium signaling; drug discovery; drug screening; high-throughput screening; mitochondria; mitochondrial calcium uniporter.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Alonso MT, Navas-Navarro P, García-Sancho J. A microplate-based bioluminescence assay of mitochondrial calcium uptake. Methods Mol Biol. 2017;1567:245–253. - PubMed
-
- Bell DH. Characterization of the fluorescence of the antitumor agent, mitoxantrone. Biochim Biophys Acta. 1988;949:132–137. - PubMed
-
- Bonora M, Giorgi C, Bononi A, Marchi S, Patergnani S, Rimessi A, Rizzuto R, Pinton P. Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat Protoc. 2013;8:2105–2118. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

