24-h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co-suspension delivery technology in COPD
- PMID: 28821260
- PMCID: PMC5563048
- DOI: 10.1186/s12931-017-0636-4
24-h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co-suspension delivery technology in COPD
Abstract
Background: Symptoms of chronic obstructive pulmonary disease may vary throughout the day and it is important that therapeutic approaches provide 24-h symptom control. We report the results of two phase IIIb crossover studies, PT003011 and PT003012, investigating the 24-h lung function profile of GFF MDI (glycopyrrolate/formoterol fumarate 18/9.6 μg delivered using innovative co-suspension delivery technology) administered twice daily.
Methods: Patients with moderate-to-very severe chronic obstructive pulmonary disease received 4 weeks' treatment with each of GFF MDI, placebo MDI, and open-label tiotropium (PT003011 only). Lung function was assessed over 24 h on day 29 of each treatment period. The primary outcome was forced expiratory volume in 1 second area under the curve from 0 to 24 h (FEV1AUC0-24). Other outcomes included change from baseline in average daily rescue medication use over the treatment period. In addition, we conducted a post-hoc analysis of data pooled from both studies to further characterize the effect of GFF MDI on inspiratory capacity.
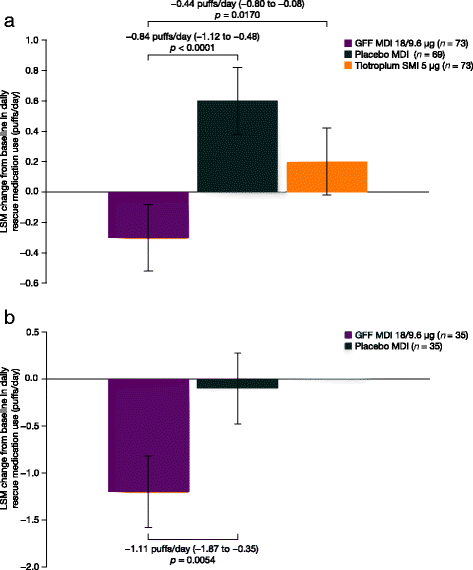
Results: GFF MDI treatment significantly increased FEV1AUC0-24 versus placebo in studies PT003011 (n = 75) and PT003012 (n = 35) on day 29 (both studies p < 0.0001), with similar improvements in FEV1AUC versus placebo for hours 0-12 and 12-24. In PT003011, improvements with GFF MDI versus tiotropium in FEV1AUC were greater during hours 12-24 compared to 0-12 h. GFF MDI treatment also resulted in a significant reduction in rescue medication use versus placebo (-0.84 [p<0.0001] and -1.11 [p=0.0054] puffs/day in PT003011 and PT003012, respectively), and versus tiotropium in PT003011 (-0.44 [p=0.017] puffs/day). A post-hoc pooled analysis showed patients treated with GFF MDI were more likely to achieve a >15% increase from baseline in inspiratory capacity than patients treated with placebo or tiotropium (72.1%, 19.0% and 47.0% of patients, respectively after the evening dose on day 29). There were no significant safety/tolerability findings.
Conclusions: GFF MDI significantly improved 24-h lung function versus placebo in patients with moderate-to-very severe chronic obstructive pulmonary disease, with similar benefits in the second 12-h period compared to the first, supporting twice-daily dosing of GFF MDI.
Trial registration: Pearl Therapeutics, Inc.; www.clinicaltrials.gov ; NCT02347072 and NCT02347085 . Registered 21 January 2015.
Keywords: Bronchodilator; COPD; Chronic bronchitis; Co-suspension delivery technology; Emphysema; Metered dose inhaler; Muscarinic antagonists; Smoking; β2-agonist.
Conflict of interest statement
Ethics approval and consent to participate
These studies were conducted in accordance with Good Clinical Practice guidelines including the International Council on Harmonisation, the US Code of Federal Regulations, and the Declaration of Helsinki. All patients provided written informed consent prior to the performance of any screening evaluations.
Consent for publication
Not applicable
Competing interests
CR and SS are employees of AstraZeneca.
AK was the Principal Investigator at Palmetto Medical Research Associates, LLC for this clinical research trial, and was paid a fee for his activities as an investigator. He is also a part owner of this research site.
JK reports personal fees from AstraZeneca, outside of the submitted work.
CO is a former employee of Pearl Therapeutics, Inc., and reports receiving personal fees from Pearl Therapeutics, Inc. outside of the submitted work.
AM and ESR are employees of Pearl Therapeutics, Inc.
UM is an employee of and holds shares in AstraZeneca.
GG, FF, LD, SA, GF, and KP have no potential competing interests to disclose.
Bevespi Aerosphere™ is a trademark of the AstraZeneca group of companies.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD, GOLD. 2017. http://www.goldcopd.org. Accessed 7 Apr 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

