Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury
- PMID: 28821269
- PMCID: PMC5563003
- DOI: 10.1186/s12974-017-0933-3
Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury
Abstract
Background: Spinal cord injury (SCI) among people over age 40 has been steadily increasing since the 1980s and is associated with worsened outcome than injuries in young people. Age-related increases in reactive oxygen species (ROS) are suggested to lead to chronic inflammation. The NADPH oxidase 2 (NOX2) enzyme is expressed by microglia and is a primary source of ROS. This study aimed to determine the effect of age on inflammation, oxidative damage, NOX2 gene expression, and functional performance with and without SCI in young adult (3 months) and middle-aged (12 months) male rats.
Methods: Young adult and middle-aged rats were assessed in two groups-naïve and moderate contusion SCI. Functional recovery was determined by weekly assessment with the Basso, Beattie, and Breshnahan general motor score (analyzed two-way ANOVA) and footprint analysis (analyzed by Chi-square analysis). Tissue was analyzed for markers of oxidative damage (8-OHdG, Oxyblot, and 3-NT), microglial-related inflammation (Iba1), NOX2 component (p47PHOX, p22PHOX, and gp91PHOX), and inflammatory (CD86, CD206, TNFα, and NFκB) gene expression (all analyzed by unpaired Student's t test).
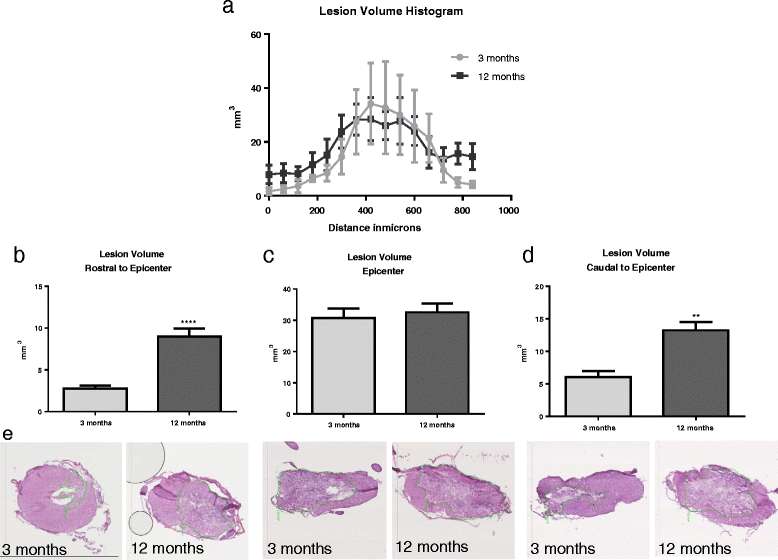
Results: In both naïve and injured aged rats, compared to young rats, tissue analysis revealed significant increases in 8-OHdG and Iba1, as well as inflammatory and NOX2 component gene expression. Further, injured aged rats showed greater lesion volume rostral and caudal to the injury epicenter. Finally, injured aged rats showed significantly reduced Basso-Beattie-Bresnahan (BBB) scores and stride length after SCI.
Conclusions: These results show that middle-aged rats demonstrate increased microglial activation, oxidative stress, and inflammatory gene expression, which may be related to elevated NOX2 expression, and contribute to worsened functional outcome following injury. These findings are essential to elucidating the mechanisms of age-related differences in response to SCI and developing age-appropriate therapeutics.
Keywords: Aging; Inflammation; Microglia; NOX2; Spinal cord injury.
Conflict of interest statement
Ethics approval
All animal procedures were approved by the Uniformed Services University IACUC and complied fully with the principles set forth in the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (DHEW pub. No. (NIH) 85-23, 2985).
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- NSCISC . National Spinal Cord Injury Statistical Center, facts and figures at a glance. Birmingham: University of Alabama at Birmingham; 2016. p. 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

