Genetic deletion of mGlu2 metabotropic glutamate receptors improves the short-term outcome of cerebral transient focal ischemia
- PMID: 28821279
- PMCID: PMC5562974
- DOI: 10.1186/s13041-017-0319-6
Genetic deletion of mGlu2 metabotropic glutamate receptors improves the short-term outcome of cerebral transient focal ischemia
Abstract
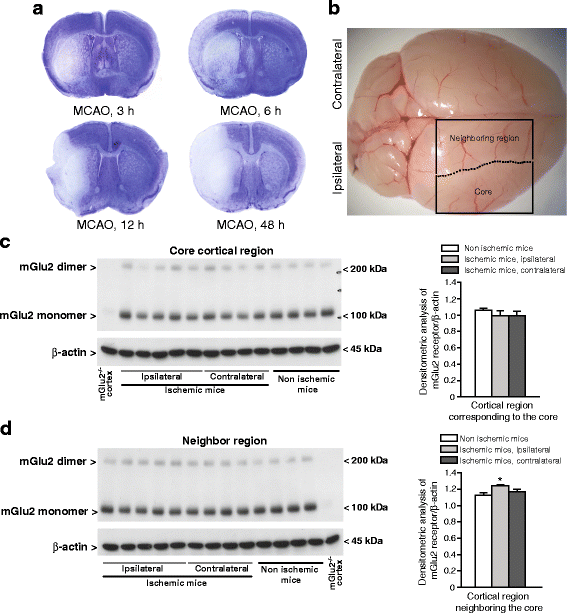
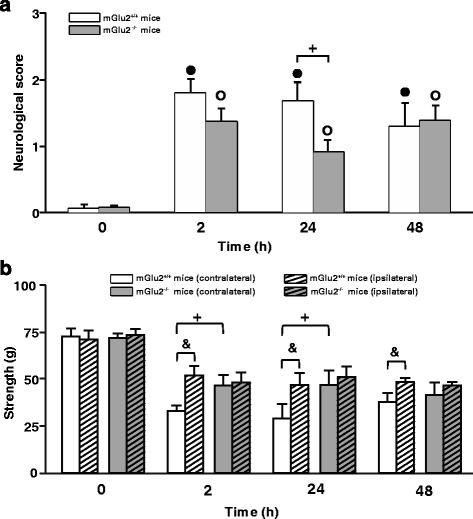
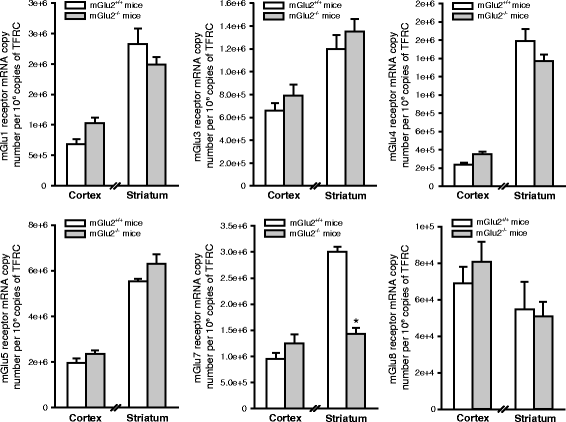
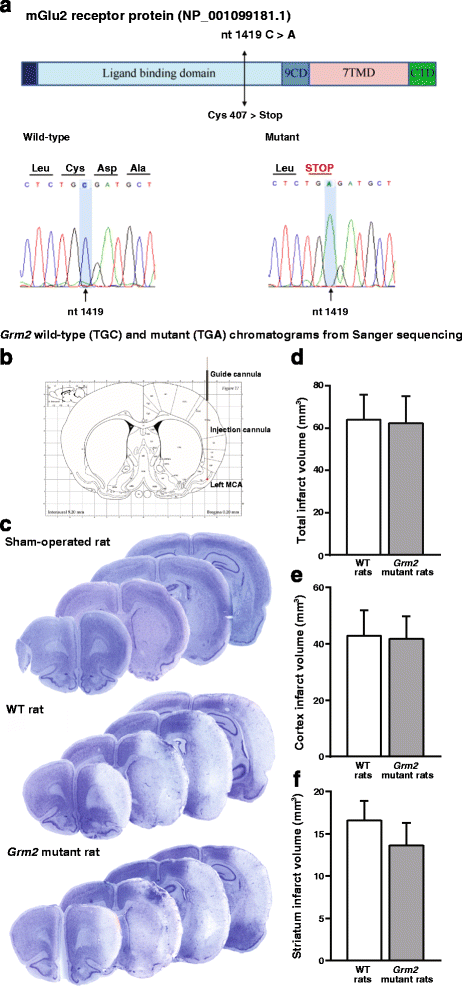
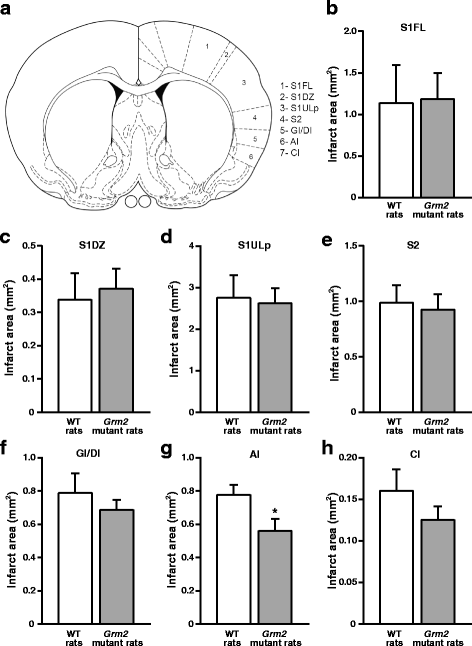
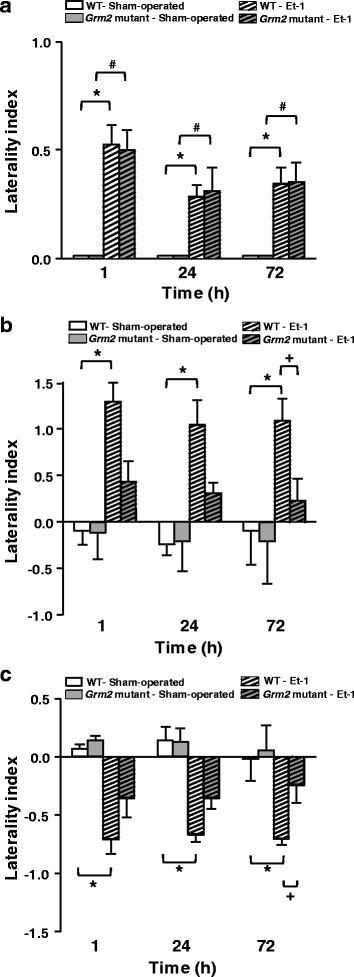
We have recently shown that pharmacological blockade of mGlu2 metabotropic glutamate receptors protects vulnerable neurons in the 4-vessel occlusion model of transient global ischemia, whereas receptor activation amplifies neuronal death. This raised the possibility that endogenous activation of mGlu2 receptors contributes to the pathophysiology of ischemic neuronal damage. Here, we examined this possibility using two models of transient focal ischemia: (i) the monofilament model of middle cerebral artery occlusion (MCAO) in mice, and (ii) the model based on intracerebral infusion of endothelin-1 (Et-1) in rats. Following transient MCAO, mGlu2 receptor knockout mice showed a significant reduction in infarct volume and an improved short-term behavioural outcome, as assessed by a neurological disability scale and the "grip test". Following Et-1 infusion, Grm2 gene mutated Hannover Wistar rats lacking mGlu2 receptors did not show changes in the overall infarct volume as compared to their wild-type counterparts, although they showed a reduced infarct area in the agranular insular cortex. Interestingly, however, mGlu2 receptor-deficient rats performed better than wild-type rats in the adhesive tape test, in which these rats did not show the laterality preference typically observed after focal ischemia. These findings support the hypothesis that activation of mGlu2 receptors is detrimental in the post-ischemic phase, and support the use of mGlu2 receptor antagonists in the experimental treatment of brain ischemia.
Keywords: Focal ischemia; Genetic deletion; Neurological score; Neuroprotection; mGlu2 receptor.
Conflict of interest statement
Ethics approval and consent to participate
Studies involving animals were performed in agreement with the National and International guidelines and regulations on animal care and use, and were approved by the Neuromed Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Consent for publication
Not applicable.
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, et al. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate recptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27(31):8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. - DOI - PMC - PubMed
-
- Caraci F, Molinaro G, Battaglia G, Giuffrida ML, Riozzi B, Traficante A, et al. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol. 2011;79(3):618–626. doi: 10.1124/mol.110.067488. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

