Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials
- PMID: 28821519
- PMCID: PMC5629648
- DOI: 10.1136/bmjopen-2017-015867
Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials
Abstract
Objective: Due to long lag time between infection/cancer diagnoses human papillomavirus (HPV) vaccination programs will deliver vaccine efficacy (VE) estimates against cancer end-points late. Cancer registry follow-up of population-based, randomised trial cohorts of vaccinated and unvaccinated women was undertaken for the estimation of VE against cervical intraepithelial neoplasia grade three and invasive cancer (CIN3+).
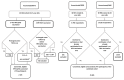
Methods: We report interim results with 98 561 person years of Finnish Cancer Registry -based follow-up of individually and/or cluster randomised cohorts of HPV-16/18 vaccinated and unvaccinated adolescent women enrolled in June 2003/2005, and between May 2004 and April 2005, respectively. The cohorts comprised 15 627 18- to 19-year-old unvaccinated women (NCT01393470), and 2 401 and 64 16- to 17-year-old HPV-16/18 vaccinated women participating the PATRICIA (NCT00122681) and HPV-012 (NCT00169494) trials, respectively. The age-aligned passive follow-up started 6 months after the clinical trials' end.
Results: During the follow-up of 4.5 to 10 years post enrolment we identified 75 cases of cervical intraepithelial neoplasia grade 3 (CIN3) and 4 cases of invasive cervical cancer (ICC) in the unvaccinated cohort, and 4 CIN3 cases in the HPV-16/18 vaccinated women. Diagnostic blocks were available for HPV typing from 87% of the cases. CIN3+ lesions were detectable in 54 cases. HPV16 was found in 26 of 50 unvaccinated CIN3+ cases, and in 3 CIN3+ cases in the HPV-16/18 vaccinated women. The latter were all baseline positive for cervical HPV16 DNA. Baseline data was not available for the unvaccinated women. Intention-to-treat VE against any CIN3+ was 66% (95% CI 8, 88).
Conclusions: Ten years post vaccination the AS04-adjuvanted HPV-16/18 vaccine shows continued efficacy against CIN3+ irrespectively of HPV type. Vaccine efficacy was not observed in baseline HPV16 DNA positive subjects.
Trial registration number: NCT01393470.
Keywords: HPV vaccines; cervical cancer; cin3+; long-term follow-up; randomized controlled trial; vaccine efficacy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: DA, ML, JP and JD have received grants from GSK group of companies and/or Merck & Co. Inc. through their employers (DA, Family Federation Finland; ML; University of Tampere; JP and PN University of Helsinki; JD and ML, Karolinska Institute) for HPV vaccination studies. GD was and FS is employee of GSK group of companies. FS has received shares and stock options from the GSK group of companies.
Figures
References
-
- Lehtinen M, Paavonen J, Wheeler C, et al. . Overall efficacy of HPV-16/18 vaccine against the most stringent cervical pre-cancer end-points: end-of study report of a double blind, randomized trial. Lancet Oncol 2012;13:89–99. - PubMed
-
- Lehtinen M, Dillner J. Clinical HPV vaccine trials and beyond. Nature Rev Clin Oncol 2013;10:400–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous