Targeting miR-423-5p Reverses Exercise Training-Induced HCN4 Channel Remodeling and Sinus Bradycardia
- PMID: 28821541
- PMCID: PMC5636198
- DOI: 10.1161/CIRCRESAHA.117.311607
Targeting miR-423-5p Reverses Exercise Training-Induced HCN4 Channel Remodeling and Sinus Bradycardia
Abstract
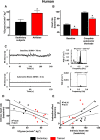
Rationale: Downregulation of the pacemaking ion channel, HCN4 (hyperpolarization-activated cyclic nucleotide gated channel 4), and the corresponding ionic current, If, underlies exercise training-induced sinus bradycardia in rodents. If this occurs in humans, it could explain the increased incidence of bradyarrhythmias in veteran athletes, and it will be important to understand the underlying processes.
Objective: To test the role of HCN4 in the training-induced bradycardia in human athletes and investigate the role of microRNAs (miRs) in the repression of HCN4.
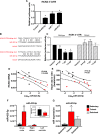
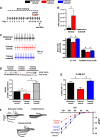
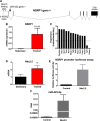
Methods and results: As in rodents, the intrinsic heart rate was significantly lower in human athletes than in nonathletes, and in all subjects, the rate-lowering effect of the HCN selective blocker, ivabradine, was significantly correlated with the intrinsic heart rate, consistent with HCN repression in athletes. Next-generation sequencing and quantitative real-time reverse transcription polymerase chain reaction showed remodeling of miRs in the sinus node of swim-trained mice. Computational predictions highlighted a prominent role for miR-423-5p. Interaction between miR-423-5p and HCN4 was confirmed by a dose-dependent reduction in HCN4 3'-untranslated region luciferase reporter activity on cotransfection with precursor miR-423-5p (abolished by mutation of predicted recognition elements). Knockdown of miR-423-5p with anti-miR-423-5p reversed training-induced bradycardia via rescue of HCN4 and If. Further experiments showed that in the sinus node of swim-trained mice, upregulation of miR-423-5p (intronic miR) and its host gene, NSRP1, is driven by an upregulation of the transcription factor Nkx2.5.
Conclusions: HCN remodeling likely occurs in human athletes, as well as in rodent models. miR-423-5p contributes to training-induced bradycardia by targeting HCN4. This work presents the first evidence of miR control of HCN4 and heart rate. miR-423-5p could be a therapeutic target for pathological sinus node dysfunction in veteran athletes.
Keywords: athletes; exercise training; ion channel remodeling; micro-RNAs; sinoatrial node; sinus bradycardia.
© 2017 The Authors.
Figures

Comment in
-
Novel Pathways for Regulation of Sinoatrial Node Plasticity and Heart Rate.Circ Res. 2017 Oct 13;121(9):1027-1028. doi: 10.1161/CIRCRESAHA.117.311922. Circ Res. 2017. PMID: 29025753 Free PMC article. No abstract available.
References
-
- Link MS, Homoud MK, Wang PJ, Estes NA., III Cardiac arrhythmias in the athlete: the evolving role of electrophysiology. Curr Sports Med Rep. 2002;1:75–85. - PubMed
-
- Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B, Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. - PubMed
-
- D’Souza A, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM, Boyett MR. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun. 2014;5:3775. doi: 10.1038/ncomms4775. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

