Cox16 protein is physically associated with Cox1p assembly intermediates and with cytochrome oxidase
- PMID: 28821616
- PMCID: PMC5625057
- DOI: 10.1074/jbc.M117.801811
Cox16 protein is physically associated with Cox1p assembly intermediates and with cytochrome oxidase
Abstract
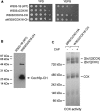
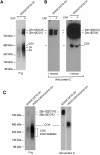
Mitochondrial cytochrome oxidase (COX) catalyzes the last step in the respiratory pathway. In the yeast Saccharomyces cerevisiae, this inner membrane complex is composed of 11 protein subunits. Expression of COX is assisted by some two dozen ancillary proteins that intercede at different stages of the assembly pathway. One such protein, Cox16p, encoded by COX16, was shown to be essential for the activity and assembly of COX. The function of Cox16p, however, has not been determined. We present evidence that Cox16p is present in Cox1p assembly intermediates and in COX. This is based on the finding that Cox16p, tagged with a dual polyhistidine and protein C tag, co-immunopurified with Cox1p assembly intermediates. The pulldown assays also indicated the presence of Cox16p in mature COX and in supercomplexes consisting of COX and the bc1 complex. From the Western signal strengths, Cox16p appears to be substoichiometric with Cox1p and Cox4p, which could indicate that Cox16p is only present in a fraction of COX. In conclusion, our results indicate that Cox16p is a constituent of several Cox1p assembly intermediates and of COX.
Keywords: cytochrome c oxidase (complex IV); electron transfer complex; membrane biogenesis; mitochondria; yeast.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., and Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272, 1136–1144 - PubMed
-
- Levchenko M., Wuttke J. M., Römpler K., Schmidt B., Neifer K., Juris L., Wissel M., Rehling P., and Deckers M. (2016) Cox26 is a novel stoichiometric subunit of the yeast cytochrome c oxidase. Biochim. Biophys. Acta 1863, 1624–1632 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

