Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin
- PMID: 28821617
- PMCID: PMC5633104
- DOI: 10.1074/jbc.M117.811802
Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin
Abstract
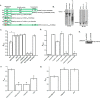
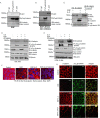
Compromise in adherens junctions (AJs) is associated with several chronic inflammatory diseases. We reported previously that Janus kinase 3, a non-receptor tyrosine kinase, plays a crucial role in AJ formation through its interaction with β-catenin. In this report, we characterize the structural determinants responsible for Jak3 interactions with β-catenin and determine the functional implications of previously unknown tyrosine residues on β-catenin phosphorylated by Jak3. We demonstrate that Jak3 autophosphorylation was the rate-limiting step during Jak3 trans-phosphorylation of β-catenin, where Jak3 directly phosphorylated three tyrosine residues, viz. Tyr30, Tyr64, and Tyr86 in the N-terminal domain (NTD) of β-catenin. However, prior phosphorylation of β-catenin at Tyr654 was essential for further phosphorylation of β-catenin by Jak3. Interaction studies indicated that phosphorylated Jak3 bound to phosphorylated β-catenin with a dissociation constant of 0.28 μm, and although both the kinase and FERM (Band
Keywords: JAK; adherens junction; barrier functions; epithelial–mesenchymal transition (EMT); mucosal immunology; protein structure function; β-catenin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures



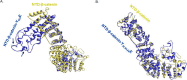
References
-
- Padmanabhan A., Rao M. V., Wu Y., and Zaidel-Bar R. (2015) Jack of all trades: functional modularity in the adherens junction. Curr. Opin. Cell Biol. 36, 32–40 - PubMed
-
- Mehta S., Nijhuis A., Kumagai T., Lindsay J., and Silver A. (2015) Defects in the adherens junction complex (E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue Res. 360, 749–760 - PubMed
-
- Alves C. H., Pellissier L. P., and Wijnholds J. (2014) The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog. Retin. Eye Res. 40, 35–52 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

