Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions
- PMID: 28821621
- PMCID: PMC5625041
- DOI: 10.1074/jbc.M117.797563
Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions
Abstract
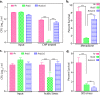
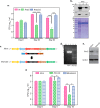
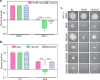
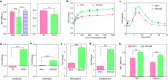
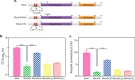
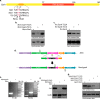
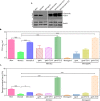
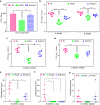
Protein kinase G (PknG), a thioredoxin-fold-containing eukaryotic-like serine/threonine protein kinase, is a virulence factor in Mycobacterium tuberculosis, required for inhibition of phagolysosomal fusion. Here, we unraveled novel functional facets of PknG during latency-like conditions. We found that PknG mediates persistence under stressful conditions like hypoxia and abets drug tolerance. PknG mutant displayed minimal growth in nutrient-limited conditions, suggesting its role in modulating cellular metabolism. Intracellular metabolic profiling revealed that PknG is necessary for efficient metabolic adaptation during hypoxia. Notably, the PknG mutant exhibited a reductive shift in mycothiol redox potential and compromised stress response. Exposure to antibiotics and hypoxic environment resulted in higher oxidative shift in mycothiol redox potential of PknG mutant compared with the wild type. Persistence during latency-like conditions required kinase activity and thioredoxin motifs of PknG and is mediated through phosphorylation of a central metabolic regulator GarA. Finally, using a guinea pig model of infection, we assessed the in vivo role of PknG in manifestation of disease pathology and established a role for PknG in the formation of stable granuloma, hallmark structures of latent tuberculosis. Taken together, PknG-mediated GarA phosphorylation is important for maintenance of both mycobacterial physiology and redox poise, an axis that is dispensable for survival under normoxic conditions but is critical for non-replicating persistence of mycobacteria. In conclusion, we propose that PknG probably acts as a modulator of latency-associated signals.
Keywords: Mycobacterium tuberculosis; hypoxia; mycobacteria; redox signaling; serine/threonine protein kinase; thioredoxin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Wayne L. G. (1976) Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am. Rev. Respir. Dis. 114, 807–811 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

