The replication and transcription activator of murine gammaherpesvirus 68 cooperatively enhances cytokine-activated, STAT3-mediated gene expression
- PMID: 28821622
- PMCID: PMC5625055
- DOI: 10.1074/jbc.M117.786970
The replication and transcription activator of murine gammaherpesvirus 68 cooperatively enhances cytokine-activated, STAT3-mediated gene expression
Abstract
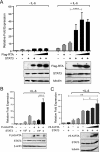
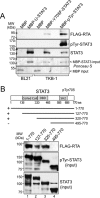
Gammaherpesviruses (γHVs) have a dynamic strategy for lifelong persistence, involving productive infection, latency, and intermittent reactivation. In latency reservoirs, such as B lymphocytes, γHVs exist as viral episomes and express few viral genes. Although the ability of γHV to reactivate from latency and re-enter the lytic phase is challenging to investigate and control, it is known that the γHV replication and transcription activator (RTA) can promote lytic reactivation. In this study, we provide first evidence that RTA of murine γΗV68 (MHV68) selectively binds and enhances the activity of tyrosine-phosphorylated host STAT3. STAT3 is a transcription factor classically activated by specific tyrosine 705 phosphorylation (pTyr705-STAT3) in response to cytokine stimulation. pTyr705-STAT3 forms a dimer that avidly binds a consensus target site in the promoters of regulated genes, and our results indicate that RTA cooperatively enhances the ability of pTyr705-STAT3 to induce expression of a STAT3-responsive reporter gene. As indicated by coimmunoprecipitation, in latently infected B cells that are stimulated to reactivate MHV68, RTA bound specifically to endogenous pTyr705-STAT3. An in vitro binding assay confirmed that RTA selectively recognizes pTyr705-STAT3 and indicated that the C-terminal transactivation domain of RTA was required for enhancing STAT3-directed gene expression. The cooperation of these transcription factors may influence both viral and host genes. During MHV68 de novo infection, pTyr705-STAT3 promoted the temporal expression of ORF59, a viral replication protein. Our results demonstrate that MHV68 RTA specifically recognizes and recruits activated pTyr705-STAT3 during the lytic phase of infection.
Keywords: STAT3; gammaherpesvirus; gene expression; infection; signal transduction; tyrosine; tyrosine phosphorylation; virology.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Identification of alternative transcripts encoding the essential murine gammaherpesvirus lytic transactivator RTA.J Virol. 2014 May;88(10):5474-90. doi: 10.1128/JVI.03110-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574412 Free PMC article.
-
Murine Gammaherpesvirus 68 ORF48 Is an RTA-Responsive Gene Product and Functions in both Viral Lytic Replication and Latency during In Vivo Infection.J Virol. 2015 Jun;89(11):5788-800. doi: 10.1128/JVI.00406-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762743 Free PMC article.
-
Lytic Replication and Reactivation from B Cells Is Not Required for Establishing or Maintaining Gammaherpesvirus Latency In Vivo.J Virol. 2022 Jun 22;96(12):e0069022. doi: 10.1128/jvi.00690-22. Epub 2022 Jun 1. J Virol. 2022. PMID: 35647668 Free PMC article.
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
Cited by
-
Multifaceted roles for STAT3 in gammaherpesvirus latency revealed through in vivo B cell knockout models.mBio. 2024 Feb 14;15(2):e0299823. doi: 10.1128/mbio.02998-23. Epub 2024 Jan 3. mBio. 2024. PMID: 38170993 Free PMC article.
-
The Role of PARP1 in Monocyte and Macrophage Commitment and Specification: Future Perspectives and Limitations for the Treatment of Monocyte and Macrophage Relevant Diseases with PARP Inhibitors.Cells. 2020 Sep 6;9(9):2040. doi: 10.3390/cells9092040. Cells. 2020. PMID: 32900001 Free PMC article. Review.
-
Conquering the Host: Determinants of Pathogenesis Learned from Murine Gammaherpesvirus 68.Annu Rev Virol. 2021 Sep 29;8(1):349-371. doi: 10.1146/annurev-virology-011921-082615. Annu Rev Virol. 2021. PMID: 34586873 Free PMC article.
-
STAT3 is a genetic modifier of TGF-beta induced EMT in KRAS mutant pancreatic cancer.Elife. 2024 Apr 4;13:RP92559. doi: 10.7554/eLife.92559. Elife. 2024. PMID: 38573819 Free PMC article.
-
B cell-intrinsic STAT3-mediated support of latency and interferon suppression during murine gammaherpesvirus 68 infection revealed through an in vivo competition model.bioRxiv [Preprint]. 2023 Mar 22:2023.03.22.533727. doi: 10.1101/2023.03.22.533727. bioRxiv. 2023. PMID: 36993230 Free PMC article. Preprint.
References
-
- Barton E., Mandal P., and Speck S. H. (2011) Pathogenesis and host control of gammaherpesviruses: Lessons from the mouse. Annu. Rev. Immunol. 29, 351–397 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

