IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis
- PMID: 28821630
- PMCID: PMC5690386
- DOI: 10.1096/fj.201700289R
IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis
Abstract
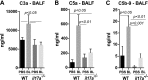
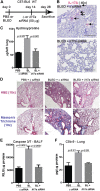
Interleukin 17A (IL-17A) and complement (C') activation have each been implicated in the pathogenesis of idiopathic pulmonary fibrosis (IPF). We have reported that IL-17A induces epithelial injury via TGF-β in murine bronchiolitis obliterans; that TGF-β and the C' cascade present signaling interactions in mediating epithelial injury; and that the blockade of C' receptors mitigates lung fibrosis. In the present study, we investigated the role of IL-17A in regulating C' in lung fibrosis. Microarray analyses of mRNA isolated from primary normal human small airway epithelial cells indicated that IL-17A (100 ng/ml; 24 h; n = 5 donor lungs) induces C' components (C' factor B, C3, and GPCR kinase isoform 5), cytokines (IL8, -6, and -1B), and cytokine ligands (CXCL1, -2, -3, -5, -6, and -16). IL-17A induces protein and mRNA regulation of C' components and the synthesis of active C' 3a (C3a) in normal primary human alveolar type II epithelial cells (AECs). Wild-type mice subjected to IL-17A neutralization and IL-17A knockout (il17a-/- ) mice were protected against bleomycin (BLEO)-induced fibrosis and collagen deposition. Further, BLEO-injured il17a-/- mice had diminished levels of circulating Krebs Von Den Lungen 6 (alveolar epithelial injury marker), local caspase-3/7, and local endoplasmic reticular stress-related genes. BLEO-induced local C' activation [C3a, C5a, and terminal C' complex (C5b-9)] was attenuated in il17a-/- mice, and IL-17A neutralization prevented the loss of epithelial C' inhibitors (C' receptor-1 related isoform Y and decay accelerating factor), and an increase in local TUNEL levels. RNAi-mediated gene silencing of il17a in fibrotic mice arrested the progression of lung fibrosis, attenuated cellular apoptosis (caspase-3/7) and lung deposition of collagen and C' (C5b-9). Compared to normals, plasma from IPF patients showed significantly higher hemolytic activity. Our findings demonstrate that limiting complement activation by neutralizing IL-17A is a potential mechanism in ameliorating lung fibrosis.-Cipolla, E., Fisher, A. J., Gu, H., Mickler, E. A., Agarwal, M., Wilke, C. A., Kim, K. K., Moore, B. B., Vittal, R. IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis.
Keywords: C3a; C5a; C5b-9; DAF; ER stress.
© FASEB.
Figures





Similar articles
-
Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis.FASEB J. 2016 Jun;30(6):2336-50. doi: 10.1096/fj.201500044. Epub 2016 Mar 8. FASEB J. 2016. PMID: 26956419 Free PMC article.
-
Overexpression of Decay Accelerating Factor Mitigates Fibrotic Responses to Lung Injury.Am J Respir Cell Mol Biol. 2022 Oct;67(4):459-470. doi: 10.1165/rcmb.2021-0463OC. Am J Respir Cell Mol Biol. 2022. PMID: 35895592 Free PMC article.
-
Crosstalk between TGF-β1 and complement activation augments epithelial injury in pulmonary fibrosis.FASEB J. 2014 Oct;28(10):4223-34. doi: 10.1096/fj.13-247650. Epub 2014 Jun 23. FASEB J. 2014. PMID: 24958208 Free PMC article.
-
Acute Lung Injury: IL-17A-Mediated Inflammatory Pathway and Its Regulation by Curcumin.Inflammation. 2019 Aug;42(4):1160-1169. doi: 10.1007/s10753-019-01010-4. Inflammation. 2019. PMID: 31011925 Review.
-
Pulmonary fibrosis and type-17 immunity.Respir Investig. 2023 Sep;61(5):553-562. doi: 10.1016/j.resinv.2023.05.005. Epub 2023 Jun 23. Respir Investig. 2023. PMID: 37356133 Review.
Cited by
-
IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a.Sci Signal. 2018 Oct 9;11(551):eaat4617. doi: 10.1126/scisignal.aat4617. Sci Signal. 2018. PMID: 30301788 Free PMC article.
-
Interleukin-17 pathways in systemic sclerosis-associated fibrosis.Rheumatol Int. 2019 Jul;39(7):1135-1143. doi: 10.1007/s00296-019-04317-5. Epub 2019 May 9. Rheumatol Int. 2019. PMID: 31073660 Review.
-
Monocyte upregulation of podoplanin during early sepsis induces complement inhibitor release to protect liver function.JCI Insight. 2020 Jul 9;5(13):e134749. doi: 10.1172/jci.insight.134749. JCI Insight. 2020. PMID: 32641582 Free PMC article.
-
Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis.Mil Med Res. 2022 May 12;9(1):21. doi: 10.1186/s40779-022-00382-3. Mil Med Res. 2022. PMID: 35550651 Free PMC article. Review.
-
Externalized histones fuel pulmonary fibrosis via a platelet-macrophage circuit of TGFβ1 and IL-27.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2215421120. doi: 10.1073/pnas.2215421120. Epub 2023 Sep 27. Proc Natl Acad Sci U S A. 2023. PMID: 37756334 Free PMC article.
References
-
- Vittal R., Fan L., Greenspan D. S., Mickler E. A., Gopalakrishnan B., Gu H., Benson H. L., Zhang C., Burlingham W., Cummings O. W., Wilkes D. S. (2013) IL-17 induces type V collagen overexpression and EMT via TGF-β-dependent pathways in obliterative bronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L401–L414 - PMC - PubMed
-
- Fan L., Benson H. L., Vittal R., Mickler E. A., Presson R., Fisher A. J., Cummings O. W., Heidler K. M., Keller M. R., Burlingham W. J., Wilkes D. S. (2011) Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am. J. Transplant. 11, 911–922 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

