Calpain-Dependent Degradation of Nucleoporins Contributes to Motor Neuron Death in a Mouse Model of Chronic Excitotoxicity
- PMID: 28821644
- PMCID: PMC6596668
- DOI: 10.1523/JNEUROSCI.0730-17.2017
Calpain-Dependent Degradation of Nucleoporins Contributes to Motor Neuron Death in a Mouse Model of Chronic Excitotoxicity
Abstract
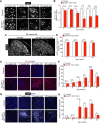
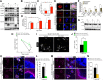
Glutamate-mediated excitotoxicity induces neuronal death by altering various intracellular signaling pathways and is implicated as a common pathogenic pathway in many neurodegenerative diseases. In the case of motor neuron disease, there is significant evidence to suggest that the overactivation of AMPA receptors due to deficiencies in the expression and function of glial glutamate transporters GLT1 and GLAST plays an important role in the mechanisms of neuronal death. However, a causal role for glial glutamate transporter dysfunction in motor neuron death remains unknown. Here, we developed a new animal model of excitotoxicity by conditionally deleting astroglial glutamate transporters GLT1 and GLAST in the spinal cords of mice (GLAST+/-/GLT1-cKO). GLAST+/-/GLT1-cKO mice (both sexes) exhibited nuclear irregularity and calpain-mediated degradation of nuclear pore complexes (NPCs), which are responsible for nucleocytoplasmic transport. These abnormalities were associated with progressive motor neuron loss, severe paralysis, and shortened lifespan. The nuclear export inhibitor KPT-350 slowed but did not prevent motor neuron death, whereas long-term treatment of the AMPA receptor antagonist perampanel and the calpain inhibitor SNJ-1945 had more persistent beneficial effects. Thus, NPC degradation contributes to AMPA receptor-mediated excitotoxic motor neuronal death, and preventing NPC degradation has robust protective effects. Normalization of NPC function could be a novel therapeutic strategy for neurodegenerative disorders in which AMPA receptor-mediated excitotoxicity is a contributory factor.SIGNIFICANCE STATEMENT Despite glial glutamate transporter dysfunction leading to excitotoxicity has been documented in many neurological diseases, it remains unclear whether its dysfunction is a primary cause or secondary outcome of neuronal death at disease state. Here we show the combined loss of glial glutamate transporters GLT1 and GLAST in spinal cord caused motor neuronal death and hindlimb paralysis. Further, our novel mutant exhibits the nuclear irregularities and calpain-mediated progressive nuclear pore complex degradation. Our study reveals that glial glutamate transporter dysfunction is sufficient to cause motor neuronal death in vivo.
Keywords: animal model; excitotoxicity; glutamate; motor neuron; transporter.
Copyright © 2017 the authors 0270-6474/17/378831-15$15.00/0.
Figures






References
-
- Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, Yanagisawa M, Nagai T, Takata N, Tanaka KF, Takayanagi R, Kano M, Götz M, Hirase H, Tanaka K (2015) Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsycopharmacology 40:1569–1579. 10.1038/npp.2015.26 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
