AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons
- PMID: 28821663
- PMCID: PMC5588461
- DOI: 10.1523/JNEUROSCI.0798-17.2017
AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons
Abstract
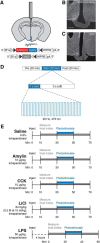
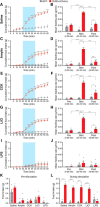
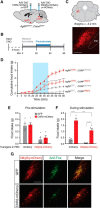
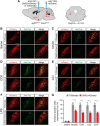
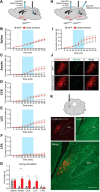
To maintain energy homeostasis, orexigenic (appetite-inducing) and anorexigenic (appetite suppressing) brain systems functionally interact to regulate food intake. Within the hypothalamus, neurons that express agouti-related protein (AgRP) sense orexigenic factors and orchestrate an increase in food-seeking behavior. In contrast, calcitonin gene-related peptide (CGRP)-expressing neurons in the parabrachial nucleus (PBN) suppress feeding. PBN CGRP neurons become active in response to anorexigenic hormones released following a meal, including amylin, secreted by the pancreas, and cholecystokinin (CCK), secreted by the small intestine. Additionally, exogenous compounds, such as lithium chloride (LiCl), a salt that creates gastric discomfort, and lipopolysaccharide (LPS), a bacterial cell wall component that induces inflammation, exert appetite-suppressing effects and activate PBN CGRP neurons. The effects of increasing the homeostatic drive to eat on feeding behavior during appetite suppressing conditions are unknown. Here, we show in mice that food deprivation or optogenetic activation of AgRP neurons induces feeding to overcome the appetite suppressing effects of amylin, CCK, and LiCl, but not LPS. AgRP neuron photostimulation can also increase feeding during chemogenetic-mediated stimulation of PBN CGRP neurons. AgRP neuron stimulation reduces Fos expression in PBN CGRP neurons across all conditions. Finally, stimulation of projections from AgRP neurons to the PBN increases feeding following administration of amylin, CCK, and LiCl, but not LPS. These results demonstrate that AgRP neurons are sufficient to increase feeding during noninflammatory-based appetite suppression and to decrease activity in anorexigenic PBN CGRP neurons, thereby increasing food intake during homeostatic need.SIGNIFICANCE STATEMENT The motivation to eat depends on the relative balance of activity in distinct brain regions that induce or suppress appetite. An abnormal amount of activity in neurons that induce appetite can cause obesity, whereas an abnormal amount of activity in neurons that suppress appetite can cause malnutrition and a severe reduction in body weight. The purpose of this study was to determine whether a population of neurons known to induce appetite ("AgRP neurons") could induce food intake to overcome appetite-suppression following administration of various appetite-suppressing compounds. We found that stimulating AgRP neurons could overcome various forms of appetite suppression and decrease neural activity in a separate population of appetite-suppressing neurons, providing new insights into how the brain regulates food intake.
Keywords: AgRP; CGRP; ChR2; appetite; food intake; parabrachial nucleus.
Copyright © 2017 the authors 0270-6474/17/378678-10$15.00/0.
Figures
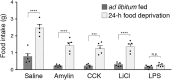

Similar articles
-
Genetically and functionally defined NTS to PBN brain circuits mediating anorexia.Nat Commun. 2016 Jun 15;7:11905. doi: 10.1038/ncomms11905. Nat Commun. 2016. PMID: 27301688 Free PMC article.
-
Hypothalamic Neurons that Regulate Feeding Can Influence Sleep/Wake States Based on Homeostatic Need.Curr Biol. 2018 Dec 3;28(23):3736-3747.e3. doi: 10.1016/j.cub.2018.09.055. Epub 2018 Nov 21. Curr Biol. 2018. PMID: 30471995 Free PMC article.
-
A discrete parasubthalamic nucleus subpopulation plays a critical role in appetite suppression.Elife. 2022 May 4;11:e75470. doi: 10.7554/eLife.75470. Elife. 2022. PMID: 35507386 Free PMC article.
-
GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism.Eur J Pharmacol. 2011 Jun 11;660(1):21-7. doi: 10.1016/j.ejphar.2010.10.110. Epub 2011 Jan 3. Eur J Pharmacol. 2011. PMID: 21211531 Free PMC article. Review.
-
Targeting AgRP neurons to maintain energy balance: Lessons from animal models.Biochem Pharmacol. 2018 Sep;155:224-232. doi: 10.1016/j.bcp.2018.07.008. Epub 2018 Jul 18. Biochem Pharmacol. 2018. PMID: 30012460 Review.
Cited by
-
Functional and Neurochemical Identification of Ghrelin Receptor (GHSR)-Expressing Cells of the Lateral Parabrachial Nucleus in Mice.Front Neurosci. 2021 Feb 15;15:633018. doi: 10.3389/fnins.2021.633018. eCollection 2021. Front Neurosci. 2021. PMID: 33658910 Free PMC article.
-
Serotonin, food intake, and obesity.Obes Rev. 2021 Jul;22(7):e13210. doi: 10.1111/obr.13210. Epub 2021 Feb 9. Obes Rev. 2021. PMID: 33559362 Free PMC article. Review.
-
d-Amphetamine and Feeding States Cohesively Affect Locomotion and Motor Neuron Response in Zebrafish Larvae.Brain Behav. 2024 Dec;14(12):e70173. doi: 10.1002/brb3.70173. Brain Behav. 2024. PMID: 39643450 Free PMC article.
-
The neuropeptide sulfakinin is a peripheral regulator of insect behavioral switch between mating and foraging.Elife. 2025 May 2;13:RP100870. doi: 10.7554/eLife.100870. Elife. 2025. PMID: 40314230 Free PMC article.
-
The Diverse Network of Brain Histamine in Feeding: Dissect its Functions in a Circuit-Specific Way.Curr Neuropharmacol. 2024;22(2):241-259. doi: 10.2174/1570159X21666221117153755. Curr Neuropharmacol. 2024. PMID: 36424776 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
