GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors
- PMID: 28821666
- PMCID: PMC6596671
- DOI: 10.1523/JNEUROSCI.2125-16.2017
GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors
Abstract
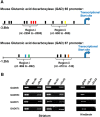
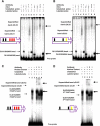
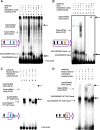
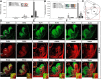
GABA is the key inhibitory neurotransmitter in the cortex but regulation of its synthesis during forebrain development is poorly understood. In the telencephalon, members of the distal-less (Dlx) homeobox gene family are expressed in, and regulate the development of, the basal ganglia primodia from which many GABAergic neurons originate and migrate to other forebrain regions. The Dlx1/Dlx2 double knock-out mice die at birth with abnormal cortical development, including loss of tangential migration of GABAergic inhibitory interneurons to the neocortex (Anderson et al., 1997a). We have discovered that specific promoter regulatory elements of glutamic acid decarboxylase isoforms (Gad1 and Gad2), which regulate GABA synthesis from the excitatory neurotransmitter glutamate, are direct transcriptional targets of both DLX1 and DLX2 homeoproteins in vivo Further gain- and loss-of-function studies in vitro and in vivo demonstrated that both DLX1 and DLX2 are necessary and sufficient for Gad gene expression. DLX1 and/or DLX2 activated the transcription of both Gad genes, and defects in Dlx function disrupted the differentiation of GABAergic interneurons with global reduction in GABA levels in the forebrains of the Dlx1/Dlx2 double knock-out mouse in vivo Identification of Gad genes as direct Dlx transcriptional targets is significant; it extends our understanding of Dlx gene function in the developing forebrain beyond the regulation of tangential interneuron migration to the differentiation of GABAergic interneurons arising from the basal telencephalon, and may help to unravel the pathogenesis of several developmental brain disorders.SIGNIFICANCE STATEMENT GABA is the major inhibitory neurotransmitter in the brain. We show that Dlx1/Dlx2 homeobox genes regulate GABA synthesis during forebrain development through direct activation of glutamic acid decarboxylase enzyme isoforms that convert glutamate to GABA. This discovery helps explain how Dlx mutations result in abnormal forebrain development, due to defective differentiation, in addition to the loss of tangential migration of GABAergic inhibitory interneurons to the neocortex. Reduced numbers or function of cortical GABAergic neurons may lead to hyperactivity states such as seizures (Cobos et al., 2005) or contribute to the pathogenesis of some autism spectrum disorders. GABAergic dysfunction in the basal ganglia could disrupt the learning and development of complex motor and cognitive behaviors (Rubenstein and Merzenich, 2003).
Keywords: GABA; forebrain development; glutamic acid decarboxylase; homeobox; interneuron.
Copyright © 2017 the authors 0270-6474/17/378817-14$15.00/0.
Figures


Similar articles
-
Dlx homeobox genes promote cortical interneuron migration from the basal forebrain by direct repression of the semaphorin receptor neuropilin-2.J Biol Chem. 2007 Jun 29;282(26):19071-81. doi: 10.1074/jbc.M607486200. Epub 2007 Jan 26. J Biol Chem. 2007. PMID: 17259176
-
Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina.J Comp Neurol. 2003 Jun 23;461(2):187-204. doi: 10.1002/cne.10674. J Comp Neurol. 2003. PMID: 12724837
-
Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins.J Neurosci. 2006 May 17;26(20):5383-92. doi: 10.1523/JNEUROSCI.1262-06.2006. J Neurosci. 2006. PMID: 16707790 Free PMC article.
-
The distribution of Dlx1-2 and glutamic acid decarboxylase in the embryonic and adult hypothalamus reveals three differentiated LHA subdivisions in rodents.J Chem Neuroanat. 2022 Apr;121:102089. doi: 10.1016/j.jchemneu.2022.102089. Epub 2022 Mar 10. J Chem Neuroanat. 2022. PMID: 35283254 Review.
-
Secondary neurogenesis and telencephalic organization in zebrafish and mice: a brief review.Integr Zool. 2009 Mar;4(1):123-133. doi: 10.1111/j.1749-4877.2008.00140.x. Integr Zool. 2009. PMID: 21392282 Review.
Cited by
-
Shh activation restores interneurons and cognitive function in newborns with intraventricular haemorrhage.Brain. 2023 Feb 13;146(2):629-644. doi: 10.1093/brain/awac271. Brain. 2023. PMID: 35867870 Free PMC article.
-
DLX genes and proteins in mammalian forebrain development.Development. 2024 Jun 1;151(11):dev202684. doi: 10.1242/dev.202684. Epub 2024 May 31. Development. 2024. PMID: 38819455 Free PMC article. Review.
-
Building thalamic neuronal networks during mouse development.Front Neural Circuits. 2023 Feb 3;17:1098913. doi: 10.3389/fncir.2023.1098913. eCollection 2023. Front Neural Circuits. 2023. PMID: 36817644 Free PMC article. Review.
-
Pre-implantation alcohol exposure induces lasting sex-specific DNA methylation programming errors in the developing forebrain.Clin Epigenetics. 2021 Aug 23;13(1):164. doi: 10.1186/s13148-021-01151-0. Clin Epigenetics. 2021. PMID: 34425890 Free PMC article.
-
Genetic Regulation of Vertebrate Forebrain Development by Homeobox Genes.Front Neurosci. 2022 Apr 25;16:843794. doi: 10.3389/fnins.2022.843794. eCollection 2022. Front Neurosci. 2022. PMID: 35546872 Free PMC article. Review.
References
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL (2001) Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
