Learning and Stress Shape the Reward Response Patterns of Serotonin Neurons
- PMID: 28821671
- PMCID: PMC6596795
- DOI: 10.1523/JNEUROSCI.1181-17.2017
Learning and Stress Shape the Reward Response Patterns of Serotonin Neurons
Abstract
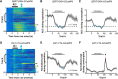
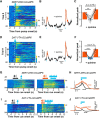
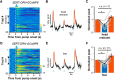
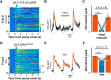
The ability to predict reward promotes animal survival. Both dopamine neurons in the ventral tegmental area and serotonin neurons in the dorsal raphe nucleus (DRN) participate in reward processing. Although the learning effects on dopamine neurons have been extensively characterized, it remains largely unknown how the response of serotonin neurons evolves during learning. Moreover, although stress is known to strongly influence reward-related behavior, we know very little about how stress modulates neuronal reward responses. By monitoring Ca2+ signals during the entire process of Pavlovian conditioning, we here show that learning differentially shapes the response patterns of serotonin neurons and dopamine neurons in mice of either sex. Serotonin neurons gradually develop a slow ramp-up response to the reward-predicting cue, and ultimately remain responsive to the reward, whereas dopamine neurons increase their response to the cue but reduce their response to the reward. For both neuron types, the responses to the cue and the reward depend on reward value, are reversible when the reward is omitted, and are rapidly reinstated by restoring the reward. We also found that stressors including head restraint and fearful context substantially reduce the response strength of both neuron types, to both the cue and the reward. These results reveal the dynamic nature of the reward responses, support the hypothesis that DRN serotonin neurons signal the current likelihood of receiving a net benefit, and suggest that the inhibitory effect of stress on the reward responses of serotonin neurons and dopamine neurons may contribute to stress-induced anhedonia.SIGNIFICANCE STATEMENT Both serotonin neurons in the dorsal raphe and dopamine neurons in the ventral tegmental area are intimately involved in reward processing. Using long-term fiber photometry of Ca2+ signals from freely behaving mice, we here show that learning produces a ramp-up activation pattern in serotonin neurons that differs from that in dopamine neurons, indicating complementary roles for these two neuron types in reward processing. Moreover, stress treatment substantially reduces the reward responses of both serotonin neurons and dopamine neurons, suggesting a possible physiological basis for stress-induced anhedonia.
Keywords: Pavlovian conditioning; fiber photometry; learning; stress-induced anhedonia; stressors; sucrose.
Copyright © 2017 the authors 0270-6474/17/378863-13$15.00/0.
Figures



Similar articles
-
Dorsal Raphe Dopamine Neurons Signal Motivational Salience Dependent on Internal State, Expectation, and Behavioral Context.J Neurosci. 2021 Mar 24;41(12):2645-2655. doi: 10.1523/JNEUROSCI.2690-20.2021. Epub 2021 Feb 9. J Neurosci. 2021. PMID: 33563725 Free PMC article.
-
Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain.Neuropharmacology. 2015 Oct;97:383-93. doi: 10.1016/j.neuropharm.2015.06.003. Epub 2015 Jun 22. Neuropharmacology. 2015. PMID: 26113399
-
Cue and Reward Evoked Dopamine Activity Is Necessary for Maintaining Learned Pavlovian Associations.J Neurosci. 2021 Jun 9;41(23):5004-5014. doi: 10.1523/JNEUROSCI.2744-20.2021. Epub 2021 Apr 22. J Neurosci. 2021. PMID: 33888609 Free PMC article.
-
Do dorsal raphe 5-HT neurons encode "beneficialness"?Neurobiol Learn Mem. 2016 Nov;135:40-49. doi: 10.1016/j.nlm.2016.08.008. Epub 2016 Aug 18. Neurobiol Learn Mem. 2016. PMID: 27544850 Review.
-
Reward processing by the dorsal raphe nucleus: 5-HT and beyond.Learn Mem. 2015 Aug 18;22(9):452-60. doi: 10.1101/lm.037317.114. Print 2015 Sep. Learn Mem. 2015. PMID: 26286655 Free PMC article. Review.
Cited by
-
Degeneracy and stability in neural circuits of dopamine and serotonin neuromodulators: A theoretical consideration.Front Comput Neurosci. 2023 Jan 25;16:950489. doi: 10.3389/fncom.2022.950489. eCollection 2022. Front Comput Neurosci. 2023. PMID: 36761394 Free PMC article.
-
Regulation of social hierarchy learning by serotonin transporter availability.Neuropsychopharmacology. 2022 Dec;47(13):2205-2212. doi: 10.1038/s41386-022-01378-2. Epub 2022 Aug 9. Neuropsychopharmacology. 2022. PMID: 35945275 Free PMC article.
-
Decision-making behaviors: weighing ethology, complexity, and sensorimotor compatibility.Curr Opin Neurobiol. 2018 Apr;49:42-50. doi: 10.1016/j.conb.2017.11.001. Epub 2017 Nov 25. Curr Opin Neurobiol. 2018. PMID: 29179005 Free PMC article. Review.
-
Nonlinear recurrent inhibition through facilitating serotonin release in the raphe.Nat Neurosci. 2025 May;28(5):1024-1037. doi: 10.1038/s41593-025-01912-7. Epub 2025 Apr 2. Nat Neurosci. 2025. PMID: 40175691
-
Stressed and wired: The effects of stress on the VTA circuits underlying motivated behavior.Curr Opin Endocr Metab Res. 2022 Oct;26:100388. doi: 10.1016/j.coemr.2022.100388. Epub 2022 Aug 7. Curr Opin Endocr Metab Res. 2022. PMID: 36406203 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
