Audiovisual Modulation in Mouse Primary Visual Cortex Depends on Cross-Modal Stimulus Configuration and Congruency
- PMID: 28821672
- PMCID: PMC6596670
- DOI: 10.1523/JNEUROSCI.0468-17.2017
Audiovisual Modulation in Mouse Primary Visual Cortex Depends on Cross-Modal Stimulus Configuration and Congruency
Abstract
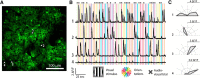
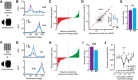
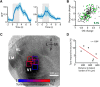
The sensory neocortex is a highly connected associative network that integrates information from multiple senses, even at the level of the primary sensory areas. Although a growing body of empirical evidence supports this view, the neural mechanisms of cross-modal integration in primary sensory areas, such as the primary visual cortex (V1), are still largely unknown. Using two-photon calcium imaging in awake mice, we show that the encoding of audiovisual stimuli in V1 neuronal populations is highly dependent on the features of the stimulus constituents. When the visual and auditory stimulus features were modulated at the same rate (i.e., temporally congruent), neurons responded with either an enhancement or suppression compared with unisensory visual stimuli, and their prevalence was balanced. Temporally incongruent tones or white-noise bursts included in audiovisual stimulus pairs resulted in predominant response suppression across the neuronal population. Visual contrast did not influence multisensory processing when the audiovisual stimulus pairs were congruent; however, when white-noise bursts were used, neurons generally showed response suppression when the visual stimulus contrast was high whereas this effect was absent when the visual contrast was low. Furthermore, a small fraction of V1 neurons, predominantly those located near the lateral border of V1, responded to sound alone. These results show that V1 is involved in the encoding of cross-modal interactions in a more versatile way than previously thought.SIGNIFICANCE STATEMENT The neural substrate of cross-modal integration is not limited to specialized cortical association areas but extends to primary sensory areas. Using two-photon imaging of large groups of neurons, we show that multisensory modulation of V1 populations is strongly determined by the individual and shared features of cross-modal stimulus constituents, such as contrast, frequency, congruency, and temporal structure. Congruent audiovisual stimulation resulted in a balanced pattern of response enhancement and suppression compared with unisensory visual stimuli, whereas incongruent or dissimilar stimuli at full contrast gave rise to a population dominated by response-suppressing neurons. Our results indicate that V1 dynamically integrates nonvisual sources of information while still attributing most of its resources to coding visual information.
Keywords: audiovisual; decoding; multisensory; neuronal ensembles; population; primary visual cortex.
Copyright © 2017 the authors 0270-6474/17/378783-14$15.00/0.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
