Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis
- PMID: 28821799
- PMCID: PMC5562746
- DOI: 10.1038/s41598-017-09114-3
Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis
Abstract
For faithful chromosome segregation, the formation of stable kinetochore-microtubule attachment and its monitoring by the spindle assembly checkpoint (SAC) are coordinately regulated by mechanisms that are currently ill-defined. Here, we show that polo-like kinase 1 (Plk1), which is instrumental in forming stable kinetochore-microtubule attachments, is also involved in the maintenance of SAC activity by binding to Bub1, but not by binding to CLASP2 or CLIP-170. The effect of Plk1 on the SAC was found to be mediated through phosphorylation of Mps1, an essential kinase for the SAC, as well as through phosphorylation of the MELT repeats in Knl1. Bub1 acts as a platform for assembling other SAC components on the phosphorylated MELT repeats. We propose that Bub1-bound Plk1 is important for the maintenance of SAC activity by supporting Bub1 localization to kinetochores in prometaphase, a time when the kinetochore Mps1 level is reduced, until the formation of stable kinetochore-microtubule attachment is completed. Our study reveals an intricate mechanism for coordinating the formation of stable kinetochore-microtubule attachment and SAC activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
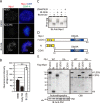

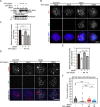
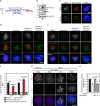

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

