Identification and analysis of brown planthopper-responsive microRNAs in resistant and susceptible rice plants
- PMID: 28821824
- PMCID: PMC5562839
- DOI: 10.1038/s41598-017-09143-y
Identification and analysis of brown planthopper-responsive microRNAs in resistant and susceptible rice plants
Abstract
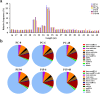
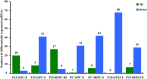
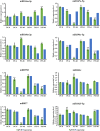
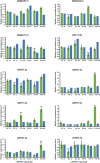
The brown planthopper (BPH) is the most devastating insect pest of rice. The rice gene BPH15 confers resistance to BPH. MicroRNAs (miRNAs) regulate a spectrum of development and defense response processes in plants. In this study, we analyzed six miRNA profiles of a BPH15 introgression line (P15) and a susceptible recipient line (PC) at three time points (0 h, 6 h and 48 h) after BPH attack, and identified 464 known miRNAs and 183 potential novel miRNAs. Before the BPH feeding, we identified 23 miRNAs differentially expressed in P15 and PC. We speculated that the resistant plant is in a priming state by the regulation of miRNAs. After the BPH feeding, 104 miRNAs were found to be expressed differentially in P15 (68 in P15-6/P15-0, 36 in P15-48/P15-0), and 80 miRNAs were found expressed differentially in PC (32 in PC-6/PC-0, 48 in PC-48/PC-0), which illustrated that miRNA expression is activated upon attack. These miRNAs regulate different pathways that contribute to the basal defense and specific resistance of rice to the BPH. Our study provides additional data for scientists to further explore the mechanism of plant defense against insect attack and to find a way for efficient insect control.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
A combined microRNA and transcriptome analyses illuminates the resistance response of rice against brown planthopper.BMC Genomics. 2020 Feb 10;21(1):144. doi: 10.1186/s12864-020-6556-6. BMC Genomics. 2020. PMID: 32041548 Free PMC article.
-
Identification and analysis of miRNAs in IR56 rice in response to BPH infestations of different virulence levels.Sci Rep. 2020 Nov 5;10(1):19093. doi: 10.1038/s41598-020-76198-9. Sci Rep. 2020. PMID: 33154527 Free PMC article.
-
BAC and RNA sequencing reveal the brown planthopper resistance gene BPH15 in a recombination cold spot that mediates a unique defense mechanism.BMC Genomics. 2014 Aug 11;15(1):674. doi: 10.1186/1471-2164-15-674. BMC Genomics. 2014. PMID: 25109872 Free PMC article.
-
Genetic and biochemical mechanisms of rice resistance to planthopper.Plant Cell Rep. 2016 Aug;35(8):1559-72. doi: 10.1007/s00299-016-1962-6. Epub 2016 Mar 15. Plant Cell Rep. 2016. PMID: 26979747 Review.
-
Insight into Rice Resistance to the Brown Planthopper: Gene Cloning, Functional Analysis, and Breeding Applications.Int J Mol Sci. 2024 Dec 13;25(24):13397. doi: 10.3390/ijms252413397. Int J Mol Sci. 2024. PMID: 39769161 Free PMC article. Review.
Cited by
-
Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review.Int J Mol Sci. 2023 Jul 27;24(15):12061. doi: 10.3390/ijms241512061. Int J Mol Sci. 2023. PMID: 37569437 Free PMC article. Review.
-
The roles of small RNAs in rice-brown planthopper interactions.Front Plant Sci. 2023 Nov 23;14:1326726. doi: 10.3389/fpls.2023.1326726. eCollection 2023. Front Plant Sci. 2023. PMID: 38078088 Free PMC article. Review.
-
Transcriptome analysis revealed differentially expressed genes in rice functionally associated with brown planthopper defense in near isogenic lines pyramiding BPH14 and BPH15.Front Plant Sci. 2023 Aug 8;14:1250590. doi: 10.3389/fpls.2023.1250590. eCollection 2023. Front Plant Sci. 2023. PMID: 37615020 Free PMC article.
-
OsmiR159 Modulate BPH Resistance Through Regulating G-Protein γ Subunit GS3 Gene in Rice.Rice (N Y). 2023 Jul 4;16(1):30. doi: 10.1186/s12284-023-00646-z. Rice (N Y). 2023. PMID: 37402009 Free PMC article.
-
Comprehensive identification and characterization of lncRNAs and circRNAs reveal potential brown planthopper-responsive ceRNA networks in rice.Front Plant Sci. 2023 Aug 10;14:1242089. doi: 10.3389/fpls.2023.1242089. eCollection 2023. Front Plant Sci. 2023. PMID: 37636117 Free PMC article.
References
-
- Engelberth J. Plant Resistance to Insect Herbivory. Biocommunication of Plants. 2012;14:303–326. doi: 10.1007/978-3-642-23524-5_16. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

