Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression
- PMID: 28821826
- PMCID: PMC5562866
- DOI: 10.1038/s41598-017-09326-7
Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression
Abstract
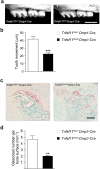
Orthodontic tooth movement is achieved by the remodeling of the alveolar bone surrounding roots of teeth. Upon the application of orthodontic force, osteoclastic bone resorption occurs on the compression side of alveolar bone, towards which the teeth are driven. However, the molecular basis for the regulatory mechanisms underlying alveolar bone remodeling has not been sufficiently elucidated. Osteoclastogenesis is regulated by receptor activator of nuclear factor-κB ligand (RANKL), which is postulated to be expressed by the cells surrounding the tooth roots. Here, we show that osteocytes are the critical source of RANKL in alveolar bone remodeling during orthodontic tooth movement. Using a newly established method for the isolation of periodontal tissue component cells from alveolar bone, we found that osteocytes expressed a much higher amount of RANKL than other cells did in periodontal tissue. The critical role of osteocyte-derived RANKL was confirmed by the reduction of orthodontic tooth movement in mice specifically lacking RANKL in osteocytes. Thus, we provide in vivo evidence for the key role of osteocyte-derived RANKL in alveolar bone remodeling, establishing a molecular basis for orthodontic force-mediated bone resorption.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

