Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice
- PMID: 28821839
- PMCID: PMC5562870
- DOI: 10.1038/s41598-017-09438-0
Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice
Abstract
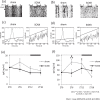
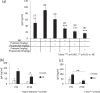
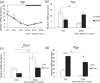
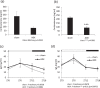
The salivary gland is rhythmically controlled by sympathetic nerve activation from the suprachiasmatic nucleus (SCN), which functions as the main oscillator of circadian rhythms. In humans, salivary IgA concentrations reflect circadian rhythmicity, which peak during sleep. However, the mechanisms controlling this rhythmicity are not well understood. Therefore, we examined whether the timing of parasympathetic (pilocarpine) or sympathetic (norepinephrine; NE) activation affects IgA secretion in the saliva. The concentrations of saliva IgA modulated by pilocarpine activation or by a combination of pilocarpine and NE activation were the highest in the middle of the light period, independent of saliva flow rate. The circadian rhythm of IgA secretion was weakened by an SCN lesion and Clock gene mutation, suggesting the importance of the SCN and Clock gene on this rhythm. Adrenoceptor antagonists blocked both NE- and pilocarpine-induced basal secretion of IgA. Dimeric IgA binds to the polymeric immunoglobulin receptor (pIgR) on the basolateral surface of epithelial cells and forms the IgA-pIgR complex. The circadian rhythm of Pigr abundance peaked during the light period, suggesting pIgR expression upon rhythmic secretion of IgA. We speculate that activation of sympathetic nerves during sleep may protect from bacterial access to the epithelial surface through enhanced secretion of IgA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Feeding and adrenal entrainment stimuli are both necessary for normal circadian oscillation of peripheral clocks in mice housed under different photoperiods.Chronobiol Int. 2015 Mar;32(2):195-210. doi: 10.3109/07420528.2014.962655. Epub 2014 Oct 6. Chronobiol Int. 2015. PMID: 25286135
-
The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock.FASEB J. 2014 Nov;28(11):4950-60. doi: 10.1096/fj.14-256594. Epub 2014 Jul 25. FASEB J. 2014. PMID: 25063847
-
Circadian rhythmicity of active GSK3 isoforms modulates molecular clock gene rhythms in the suprachiasmatic nucleus.J Biol Rhythms. 2015 Apr;30(2):155-60. doi: 10.1177/0748730415573167. Epub 2015 Feb 27. J Biol Rhythms. 2015. PMID: 25724980 Free PMC article.
-
Effect of photic stimuli on rat salivary glands. Role of sympathetic nervous system.Acta Odontol Latinoam. 2000;13(1):3-19. Acta Odontol Latinoam. 2000. PMID: 11885465 Review.
-
Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology.J Biol Rhythms. 2015 Feb;30(1):20-34. doi: 10.1177/0748730414553971. Epub 2014 Nov 3. J Biol Rhythms. 2015. PMID: 25367898 Review.
Cited by
-
Effect of Urolithin A on the Improvement of Circadian Rhythm Dysregulation in Intestinal Barrier Induced by Inflammation.Nutrients. 2024 Jul 13;16(14):2263. doi: 10.3390/nu16142263. Nutrients. 2024. PMID: 39064706 Free PMC article.
-
Daytime Exposure to Blue-Enriched Light Counters the Effects of Sleep Restriction on Cortisol, Testosterone, Alpha-Amylase and Executive Processes.Front Neurosci. 2020 Jan 8;13:1366. doi: 10.3389/fnins.2019.01366. eCollection 2019. Front Neurosci. 2020. PMID: 31998056 Free PMC article.
-
For Whom the Clock Ticks: Clinical Chronobiology for Infectious Diseases.Front Immunol. 2020 Jul 9;11:1457. doi: 10.3389/fimmu.2020.01457. eCollection 2020. Front Immunol. 2020. PMID: 32733482 Free PMC article. Review.
-
The Alteration of Salivary Immunoglobulin A in Autism Spectrum Disorders.Front Psychiatry. 2021 May 21;12:669193. doi: 10.3389/fpsyt.2021.669193. eCollection 2021. Front Psychiatry. 2021. PMID: 34093280 Free PMC article.
-
Saliva as a Diagnostic Tool for Systemic Diseases-A Narrative Review.Medicina (Kaunas). 2025 Jan 30;61(2):243. doi: 10.3390/medicina61020243. Medicina (Kaunas). 2025. PMID: 40005360 Free PMC article. Review.
References
-
- Tahara Y, Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nature reviews. Gastroenterology & hepatology. 2016;13:217–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

