The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins
- PMID: 28821884
- PMCID: PMC5562859
- DOI: 10.1038/s41598-017-09207-z
The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins
Erratum in
-
Author Correction: The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins.Sci Rep. 2018 May 29;8(1):8439. doi: 10.1038/s41598-018-26378-5. Sci Rep. 2018. PMID: 29844442 Free PMC article.
Abstract
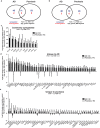
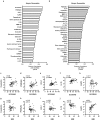
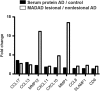
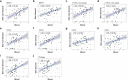
Beyond classic "allergic"/atopic comorbidities, atopic dermatitis (AD) emerges as systemic disease with increased cardiovascular risk. To better define serum inflammatory and cardiovascular risk proteins, we used an OLINK high-throughput proteomic assay to analyze moderate-to-severe AD (n = 59) compared to psoriasis (n = 22) and healthy controls (n = 18). Compared to controls, 10 proteins were increased in serum of both diseases, including Th1 (IFN-γ, CXCL9, TNF-β) and Th17 (CCL20) markers. 48 proteins each were uniquely upregulated in AD and psoriasis. Consistent with skin expression, AD serum showed up-regulation of Th2 (IL-13, CCL17, eotaxin-1/CCL11, CCL13, CCL4, IL-10), Th1 (CXCL10, CXCL11) and Th1/Th17/Th22 (IL-12/IL-23p40) responses. Surprisingly, some markers of atherosclerosis (fractalkine/CX3CL1, CCL8, M-CSF, HGF), T-cell development/activation (CD40L, IL-7, CCL25, IL-2RB, IL-15RA, CD6) and angiogenesis (VEGF-A) were significantly increased only in AD. Multiple inflammatory pathways showed stronger enrichment in AD than psoriasis. Several atherosclerosis mediators in serum (e.g. E-selectin, PI3/elafin, CCL7, IL-16) correlated with SCORAD, but not BMI. Also, AD inflammatory mediators (e.g. MMP12, IL-12/IL-23p40, CXCL9, CCL22, PI3/Elafin) correlated between blood and lesional as well as non-lesional skin. Overall, the AD blood signature was largely different compared to psoriasis, with dysregulation of inflammatory and cardiovascular risk markers, strongly supporting its systemic nature beyond atopic/allergic association.
Conflict of interest statement
PMB has received personal fees from LEO Pharma and Sanofi. MSF has received research support from Pfizer and Quorum Consulting. JGK has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. EGY is a board member for Sanofi Aventis,Regeneron,Stiefel/GlaxoSmithKline,MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira,Galderma,Glenmark,Novartis, Pfizer, Vitae, Leo Pharma, Abbvie and Asana Biosciences; has received consultancy fees from Regeneron, Sanofi, MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe, Eli Lilly, Abbvie, and Asana Biosciences; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma, Dermira, Glenmark, Innovaderm, and UCB. The rest of the authors declare that they have no relevant conflicts of interest.
Figures
Similar articles
-
The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature.J Am Acad Dermatol. 2020 Mar;82(3):690-699. doi: 10.1016/j.jaad.2019.10.039. Epub 2019 Oct 25. J Am Acad Dermatol. 2020. PMID: 31669080
-
Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities.J Allergy Clin Immunol. 2021 Apr;147(4):1369-1380. doi: 10.1016/j.jaci.2020.08.041. Epub 2020 Oct 1. J Allergy Clin Immunol. 2021. PMID: 33011244
-
Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis.Ann Allergy Asthma Immunol. 2020 Jan;124(1):70-78. doi: 10.1016/j.anai.2019.10.013. Epub 2019 Oct 14. Ann Allergy Asthma Immunol. 2020. PMID: 31622668
-
Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment.Exp Dermatol. 2018 Apr;27(4):409-417. doi: 10.1111/exd.13336. Epub 2017 May 9. Exp Dermatol. 2018. PMID: 28266782 Review.
-
Atopic dermatitis and psoriasis: two different immune diseases or one spectrum?Curr Opin Immunol. 2017 Oct;48:68-73. doi: 10.1016/j.coi.2017.08.008. Epub 2017 Sep 1. Curr Opin Immunol. 2017. PMID: 28869867 Review.
Cited by
-
Histamine and Th2 cytokines independently and synergistically upregulate MMP12 expression in human M2 macrophages.Front Immunol. 2024 Oct 21;15:1429009. doi: 10.3389/fimmu.2024.1429009. eCollection 2024. Front Immunol. 2024. PMID: 39502691 Free PMC article.
-
Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis.Nat Cardiovasc Res. 2024 Feb 1;3:221-242. doi: 10.1038/s44161-023-00413-9. Epub 2024 Jan 22. Nat Cardiovasc Res. 2024. PMID: 39044999 Free PMC article.
-
Accurate diagnosis of atopic dermatitis by combining transcriptome and microbiota data with supervised machine learning.Sci Rep. 2022 Jan 7;12(1):290. doi: 10.1038/s41598-021-04373-7. Sci Rep. 2022. PMID: 34997172 Free PMC article.
-
Biomarkers of subclinical atherosclerosis in patients with psoriasis.Sci Rep. 2021 Nov 2;11(1):21438. doi: 10.1038/s41598-021-00999-9. Sci Rep. 2021. PMID: 34728734 Free PMC article.
-
The antimicrobial protein S100A12 identified as a potential autoantigen in a subgroup of atopic dermatitis patients.Clin Transl Allergy. 2019 Jan 31;9:6. doi: 10.1186/s13601-019-0240-4. eCollection 2019. Clin Transl Allergy. 2019. PMID: 30728947 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

