Impact of processing methods on urinary biomarkers analysis in neonates
- PMID: 28821985
- PMCID: PMC5700848
- DOI: 10.1007/s00467-017-3779-0
Impact of processing methods on urinary biomarkers analysis in neonates
Abstract
Background: In neonates, the validation of urinary biomarkers to diagnose acute kidney injury is a rapidly evolving field. The neonatal population poses unique challenges when assessing the collection, storage, and processing of urinary samples for biomarker analysis. Given this, establishing optimal and consistent sample processing in this population for meaningful use in ongoing clinical trials is important.
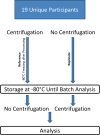
Methods: Urine from a cohort of 19 hospitalized neonatal intensive care unit patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (Clinical Trial NCT01378273) was collected for biomarker analysis by indirect techniques using Fisher-brand cotton balls placed in the diapers. Fourteen urinary biomarkers were measured using commercially available kits via electrochemiluminescence on multiarray plates and compared between paired samples processed with centrifugation prior to storage versus prior to analysis.
Results: None of the biomarker concentrations differed between samples undergoing centrifugation prior to storage versus prior to analysis. The difference between samples was within 2% of the estimated concentration for the protein in 12 of 14 biomarkers (86%), and all paired biomarker concentrations were within 4%. The percentage error analysis did not show a difference between paired samples, with biomarker percentage errors smaller than the stated immunoassay coefficient of variance.
Conclusions: The urinary concentrations of biomarkers were comparable between paired samples, demonstrating that indirectly collected neonatal urine samples do not require centrifugation after collection and before storage. The ability to use routine urine collection and storage methods to obtain samples for subsequent quantitative immunoassay analysis should facilitate studies of newborns and young children.
Keywords: Acute kidney injury; Interleukin 18; Kidney injury molecule 1; Neonates; Neutrophil gelatinase-associated lipocalin; Urinary biomarkers; Urine storage.
Conflict of interest statement
None to declare
Figures

References
-
- Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, Jarvis WR, Pediatric Prevention Network Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821–827. - PubMed
-
- Muratore C, Dhanireddy R. Urine collection for disposable diapers in premature infants: Biochemical analysis. Clin Pediatr (Phila) 1993;32:314–315. - PubMed
-
- Starr R, Kimmel P, Bonventre J. Best Practices for Sample Storage: A Report from the Workshop on Urine Biospecimen Handling [Internet] Available from: http://www3.niddk.nih.gov/fund/other/Best_Practices_for_sample_Storage.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

