Body mass index and clinical response to intravenous or subcutaneous abatacept in patients with rheumatoid arthritis
- PMID: 28822046
- PMCID: PMC5681604
- DOI: 10.1007/s10067-017-3788-1
Body mass index and clinical response to intravenous or subcutaneous abatacept in patients with rheumatoid arthritis
Abstract
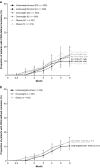
This post hoc analysis of ACQUIRE (NCT00559585) explored the effect of baseline body mass index (BMI) on the pharmacokinetics of and clinical response to subcutaneous (SC) or intravenous (IV) abatacept in patients with rheumatoid arthritis (RA). ACQUIRE was a phase 3b, 6-month, double-blind, double-dummy study in which patients with RA were randomized (1:1) to SC (fixed - dose; 125 mg/week) or IV (weight-tiered; ~ 10 mg/kg/month) abatacept plus methotrexate. In this analysis, minimum abatacept plasma concentration (Cmin) was measured at 3 and 6 months, and clinical remission over 6 months was assessed by Disease Activity Score 28 (C-reactive protein; DAS28 [CRP], < 2.6), Simplified Disease Activity Index (SDAI, ≤ 3.3), and Clinical Disease Activity Index (CDAI, ≤ 2.8). Data were stratified by baseline BMI (underweight/normal, < 25 kg/m2; overweight, 25 to < 30 kg/m2; obese, ≥ 30 kg/m2) and administration route. Of the 1456/1457 patients for whom baseline BMIs were available, 526 (36%; SC 265, IV 261) patients were underweight/normal, 497 (34%; SC 249, IV 248) were overweight, and 433 (30%; SC 221, IV 212) were obese. Median Cmin abatacept concentration was ≥ 10 μg/mL (efficacy threshold) at 3 and 6 months in > 90% of patients across BMI groups with both administration routes. DAS28 (CRP), SDAI, and CDAI remission rates at 6 months were similar across BMI groups and 95% confidence intervals overlapped at all time points in both separate and pooled SC/IV analyses. Therapeutic concentrations of abatacept and clinical remission rates using stringent criteria were similar across patient BMIs and administration routes.
Keywords: Body mass index; DAS28; Disease activity; Pharmacokinetics; Rheumatoid arthritis.
Conflict of interest statement
Disclosures
Prof. D’Agostino has received speaker’s bureau fees from Bristol-Myers Squibb, AbbVie, and Novartis, as well as research grants from Pfizer. Prof. Alten has received research grants, consulting fees, and speaker’s bureau fees from Bristol-Myers Squibb. Dr. Mysler has received research grants and speaker’s bureau fees from Bristol-Myers Squibb, Roche, Eli Lilly, AbbVie, Novartis, Janssen, and Pfizer. Dr. Le Bars and Dr. Murthy are shareholders and employees of Bristol-Myers Squibb. Dr. Ye and Dr. Vadanici are employees of Bristol-Myers Squibb. Julia Heitzmann is an employee of Excelya and a paid consultant for Bristol-Myers Squibb. Prof. Ferraccioli has received grant support from Roche and Bristol-Myers Squibb and speaker fees from Roche, Bristol-Myers Squibb, AbbVie, Pfizer, UCB, MSD, Eli Lilly, Novartis, and GlaxoSmithKline.
Funding
This study was sponsored by Bristol-Myers Squibb.
Figures



References
-
- Naranjo A, Sokka T, Descalzo MA, Calvo-Alen J, Horslev-Petersen K, Luukkainen RK, Combe B, Burmester GR, Devlin J, Ferraccioli G, Morelli A, Hoekstra M, Majdan M, Sadkiewicz S, Belmonte M, Holmqvist AC, Choy E, Tunc R, Dimic A, Bergman M, Toloza S, Pincus T. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10:R30. doi: 10.1186/ar2383. - DOI - PMC - PubMed
-
- Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, Arkema EV, Costenbader KH, Karlson EW. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014;73:1914–1922. doi: 10.1136/annrheumdis-2014-205459. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

