Current progress in innovative engineered antibodies
- PMID: 28822103
- PMCID: PMC5777977
- DOI: 10.1007/s13238-017-0457-8
Current progress in innovative engineered antibodies
Abstract
As of May 1, 2017, 74 antibody-based molecules have been approved by a regulatory authority in a major market. Additionally, there are 70 and 575 antibody-based molecules in phase III and phase I/II clinical trials, respectively. These total 719 antibody-based clinical stage molecules include 493 naked IgGs, 87 antibody-drug conjugates, 61 bispecific antibodies, 37 total Fc fusion proteins, 17 radioimmunoglobulins, 13 antibody fragments, and 11 immunocytokines. New uses for these antibodies are being discovered each year. For oncology, many of the exciting new approaches involve antibody modulation of T-cells. There are over 80 antibodies in clinical trials targeting T cell checkpoints, 26 T-cell-redirected bispecific antibodies, and 145 chimeric antigen receptor (CAR) cell-based candidates (all currently in phase I or II clinical trials), totaling more than 250 T cell interacting clinical stage antibody-based candidates. Finally, significant progress has been made recently on routes of delivery, including delivery of proteins across the blood-brain barrier, oral delivery to the gut, delivery to the cellular cytosol, and gene- and viral-based delivery of antibodies. Thus, there are currently at least 864 antibody-based clinical stage molecules or cells, with incredible diversity in how they are constructed and what activities they impart. These are followed by a next wave of novel molecules, approaches, and new methods and routes of delivery, demonstrating that the field of antibody-based biologics is very innovative and diverse in its approaches to fulfill their promise to treat unmet medical needs.
Keywords: antibody clinical candidates; chimeric antigen receptors; engineered antibodies.
Figures


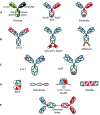
References
-
- Adam VS, Crosariol M, Kumar S, Ge MO, Czack SE, Roy S, Haczku A, Tretiakova A, Wilson JM. Adeno-associated virus 9-mediated airway expression of antibody protects old and immunodeficient mice against influenza virus. Clin Vaccine Immunol. 2014;21:1528–1533. doi: 10.1128/CVI.00572-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

