Baclofen for alcohol withdrawal
- PMID: 28822350
- PMCID: PMC6483686
- DOI: 10.1002/14651858.CD008502.pub5
Baclofen for alcohol withdrawal
Update in
-
Baclofen for alcohol withdrawal.Cochrane Database Syst Rev. 2019 Nov 6;2019(11):CD008502. doi: 10.1002/14651858.CD008502.pub6. Cochrane Database Syst Rev. 2019. PMID: 31689723 Free PMC article.
Abstract
Background: Baclofen shows potential for rapidly reducing symptoms of severe alcohol withdrawal syndrome (AWS) in people with alcoholism. Treatment with baclofen is easy to manage and rarely produces euphoria or other pleasant effects, or craving for the drug. This is an updated version of the original Cochrane Review published in 2015, Issue 4.
Objectives: To assess the efficacy and safety of baclofen for people with AWS.
Search methods: We updated our searches of the following databases to March 2017: the Cochrane Drugs and Alcohol Group Specialised Register, CENTRAL, PubMed, Embase, and CINAHL. We also searched registers of ongoing trials. We handsearched the references quoted in the identified trials, and sought information from researchers, pharmaceutical companies, and relevant trial authors about unpublished or uncompleted trials. We placed no restrictions on language.
Selection criteria: We included all randomised controlled clinical trials (RCTs) evaluating baclofen versus placebo or any other treatment for people with AWS. We excluded uncontrolled, non-randomised, or quasi-randomised trials. We included both parallel group and cross-over studies.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
Main results: We included three RCTs with 141 randomised participants. We did not perform meta-analyses due to the different control interventions. For the comparison of baclofen and placebo (1 study, 31 participants), there was no significant difference in Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) scores (very low quality evidence). For the comparison of baclofen and diazepam (1 study, 37 participants), there was no significant difference in CIWA-Ar scores (very low quality evidence), adverse events (risk difference (RD) 0.00, 95% confidence interval (CI) -0.10 to 0.10; very low quality evidence), dropouts (RD 0.00, 95% CI -0.10 to 0.10; very low quality evidence), and dropouts due to adverse events (RD 0.00, 95% CI -0.10 to 0.10; very low quality evidence). For the comparison of baclofen and chlordiazepoxide (1 study, 60 participants), there was no significant difference in CIWA-Ar scores (mean difference (MD) 1.00, 95% CI 0.70 to 1.30; very low quality evidence), global improvement (MD 0.10, 95% CI -0.03 to 0.23; very low quality evidence), adverse events (RD 2.50, 95% CI 0.88 to 7.10; very low quality of evidence), dropouts (RD 0.00, 95% CI -0.06 to 0.06; very low quality evidence), and dropouts due to adverse events (RD 0.00, 95% CI -0.06 to 0.06; very low quality evidence).
Authors' conclusions: No conclusions can be drawn about the efficacy and safety of baclofen for the management of alcohol withdrawal because we found insufficient and very low quality evidence.
Conflict of interest statement
JL: none known. LW: none known.
Figures

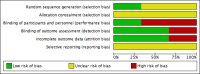


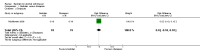
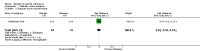
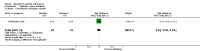




Update of
-
Baclofen for alcohol withdrawal.Cochrane Database Syst Rev. 2015 Apr 3;(4):CD008502. doi: 10.1002/14651858.CD008502.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Aug 20;8:CD008502. doi: 10.1002/14651858.CD008502.pub5. PMID: 25836263 Updated.
References
References to studies included in this review
-
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. American Journal of Medicine 2006;119(3):276.e13‐8. - PubMed
-
- Girish K, Vikram Reddy K, Pandit LV, Pundarikaksha HP, Vijendra R, Vasundara K, et al. A randomized, open‐label, standard controlled, parallel group study of efficacy and safety of baclofen, and chlordiazepoxide in uncomplicated alcohol withdrawal syndrome. Biomedical Journal 2016;39(1):72‐80. - PMC - PubMed
-
- Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM. Treating alcohol withdrawal with oral baclofen: a randomized, double‐blind, placebo‐controlled trial. Journal of Hospital Medicine 2011;6(8):469‐74. - PubMed
References to studies excluded from this review
-
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomised controlled study. Alcohol and Alcoholism (Oxford, Oxfordshire) 2002;37(5):504‐8. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind controlled study. Lancet 2007;370(9603):1915‐22. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose‐response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double‐blind, placebo‐controlled trial. Alcohol and Alcoholism (Oxford, Oxfordshire) 2011;46(3):312‐7. - PubMed
-
- Beraha EM, Salemink E, Goudriaan AE, Bakker A, Jong D, Smits N, et al. Efficacy and safety of high‐dose baclofen for the treatment of alcohol dependence: a multicentre, randomised, double‐blind controlled trial. European Neuropsychopharmacology 2016;26(12):1950‐9. - PubMed
References to ongoing studies
-
- NCT02052440. Preventing alcohol withdrawal syndrome with oral baclofen. clinicaltrials.gov/show/NCT02052440 (first received 30 January 2014).
Additional references
-
- Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. American Journal of Medicine 2002;112:226‐9. - PubMed
-
- Addolorato G, Leggio L, Abenavoli L, DeLorenzi G, Parente A, Caputo F, et al. Suppression of alcohol delirium tremens by baclofen administration: a case report. Clinical Neuropharmacology 2003;26:258‐62. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): American Psychiatric Association, 1994.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

