Spontaneous activation of a MAVS-dependent antiviral signaling pathway determines high basal interferon-β expression in cardiac myocytes
- PMID: 28822807
- PMCID: PMC5600710
- DOI: 10.1016/j.yjmcc.2017.08.008
Spontaneous activation of a MAVS-dependent antiviral signaling pathway determines high basal interferon-β expression in cardiac myocytes
Abstract
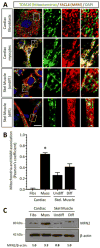
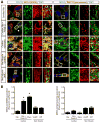
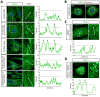
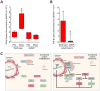
Viral myocarditis is a leading cause of sudden death in young adults as the limited turnover of cardiac myocytes renders the heart particularly vulnerable to viral damage. Viruses induce an antiviral type I interferon (IFN-α/β) response in essentially all cell types, providing an immediate innate protection. Cardiac myocytes express high basal levels of IFN-β to help pre-arm them against viral infections, however the mechanism underlying this expression remains unclear. Using primary cultures of murine cardiac and skeletal muscle cells, we demonstrate here that the mitochondrial antiviral signaling (MAVS) pathway is spontaneously activated in unstimulated cardiac myocytes but not cardiac fibroblasts or skeletal muscle cells. Results suggest that MAVS association with the mitochondrial-associated ER membranes (MAM) is a determinant of high basal IFN-β expression, and demonstrate that MAVS is essential for spontaneous high basal expression of IFN-β in cardiac myocytes and the heart. Together, results provide the first mechanism for spontaneous high expression of the antiviral cytokine IFN-β in a poorly replenished and essential cell type.
Keywords: Cardiac myocyte; Cardiomyocyte; Interferon; MAM; MAVS; Mitochondria.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




References
-
- Azuma K, Ichimura K, Mita T, Nakayama S, Jin WL, Hirose T, Fujitani Y, Sumiyoshi K, Shimada K, Daida H, Sakai T, Mitsumata M, Kawamori R, Watada H. Presence of alpha-smooth muscle actin-positive endothelial cells in the luminal surface of adult aorta. Biochem Biophys Res Commun. 2009;380:620–626. - PubMed
-
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American journal of physiology Heart and circulatory physiology. 2007;293:H1883–1891. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

