Loss of perilipin 2 in cultured myotubes enhances lipolysis and redirects the metabolic energy balance from glucose oxidation towards fatty acid oxidation
- PMID: 28822960
- PMCID: PMC5665670
- DOI: 10.1194/jlr.M079764
Loss of perilipin 2 in cultured myotubes enhances lipolysis and redirects the metabolic energy balance from glucose oxidation towards fatty acid oxidation
Abstract
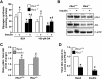
Lipid droplet (LD) coating proteins are essential for the formation and stability of intracellular LDs. Plin2 is an abundant LD coating protein in skeletal muscle, but its importance for muscle function is unclear. We show that myotubes established from Plin2-/- mice contain reduced content of LDs and accumulate less oleic acid (OA) in triacylglycerol (TAG) due to elevated LD hydrolysis in comparison with Plin2+/+ myotubes. The reduced ability to store TAG in LDs in Plin2-/- myotubes is accompanied by a shift in energy metabolism. Plin2-/- myotubes are characterized by increased oxidation of OA, lower glycogen synthesis, and reduced glucose oxidation in comparison with Plin2+/+ myotubes, perhaps reflecting competition between FAs and glucose as part of the Randle cycle. In accord with these metabolic changes, Plin2-/- myotubes have elevated expression of Ppara and Ppargc1a, transcription factors that stimulate expression of genes important for FA oxidation, whereas genes involved in glucose uptake and oxidation are suppressed. Loss of Plin2 had no impact on insulin-stimulated Akt phosphorylation. Our results suggest that Plin2 is essential for protecting the pool of skeletal muscle LDs to avoid an uncontrolled hydrolysis of stored TAG and to balance skeletal muscle energy metabolism.
Keywords: Plin2; fatty acid/metabolism; insulin signaling; lipid droplet; lipolysis and fatty acid metabolism; muscle; triacylglycerol.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Eckardt K., Taube A., and Eckel J.. 2011. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev. Endocr. Metab. Disord. 12: 163–172. - PubMed
-
- Pan D. A., Lillioja S., Kriketos A. D., Milner M. R., Baur L. A., Bogardus C., Jenkins A. B., and Storlien L. H.. 1997. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 46: 983–988. - PubMed
-
- Jacob S., Machann J., Rett K., Brechtel K., Volk A., Renn W., Maerker E., Matthaei S., Schick F., Claussen C. D., et al. . 1999. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 48: 1113–1119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

