Inhibition of P2X7 receptors improves outcomes after traumatic brain injury in rats
- PMID: 28823092
- PMCID: PMC5714842
- DOI: 10.1007/s11302-017-9579-y
Inhibition of P2X7 receptors improves outcomes after traumatic brain injury in rats
Abstract
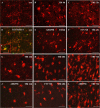
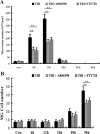
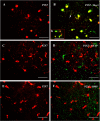
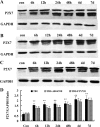
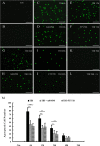
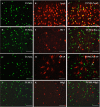
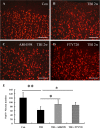
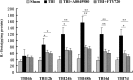
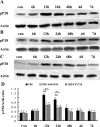
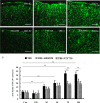
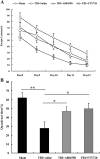
Traumatic brain injury (TBI) is the leading cause of death and disability for people under the age of 45 years worldwide. Neuropathology after TBI is the result of both the immediate impact injury and secondary injury mechanisms. Secondary injury is the result of cascade events, including glutamate excitotoxicity, calcium overloading, free radical generation, and neuroinflammation, ultimately leading to brain cell death. In this study, the P2X7 receptor (P2X7R) was detected predominately in microglia of the cerebral cortex and was up-regulated on microglial cells after TBI. The microglia transformed into amoeba-like and discharged many microvesicle (MV)-like particles in the injured and adjacent regions. A P2X7R antagonist (A804598) and an immune inhibitor (FTY720) reduced significantly the number of MV-like particles in the injured/adjacent regions and in cerebrospinal fluid, reduced the number of neurons undergoing apoptotic cell death, and increased the survival of neurons in the cerebral cortex injured and adjacent regions. Blockade of the P2X7R and FTY720 reduced interleukin-1βexpression, P38 phosphorylation, and glial activation in the cerebral cortex and improved neurobehavioral outcomes after TBI. These data indicate that MV-like particles discharged by microglia after TBI may be involved in the development of local inflammation and secondary nerve cell injury.
Keywords: Microglial cells; Microvesicles; Neuroinflammation; P2X7R; Traumatic brain injury.
Conflict of interest statement
Conflicts of interest
Xiaofeng Liu declares that she has no conflict of interest.
Zhengqing Zhao declares that she has no conflict of interest.
Ruihua Ji declares that she has no conflict of interest.
Jiao Zhu declares that she has no conflict of interest.
Qian-Qian Sui declares that she has no conflict of interest.
Gillian E. Knight declares that she has no conflict of interest.
Geoffrey Burnstock declares that he has no conflict of interest.
Cheng He declares that she has no conflict of interest.
Hongbin Yuan declares that she has no conflict of interest.
Zhenghua Xiang declares that she has no conflict of interest.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University and conformed to the UK Animals (Scientific Procedures) Act 1986 and associated guidelines on the ethical use of animals.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

